
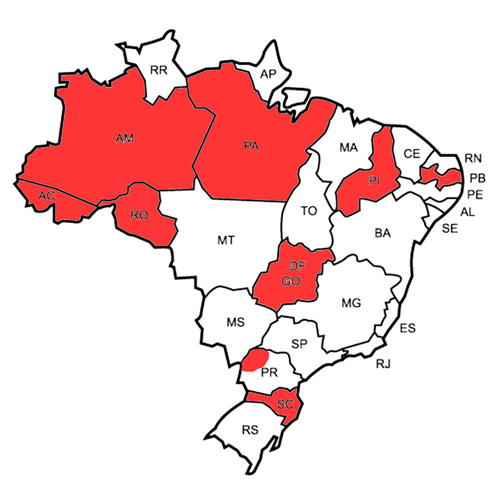
Brazilian registry of persons with hemophilia A receiving emicizumab (Emicizumab Cases, EMCase Project)
BACKGROUND Emicizumab (MC-Ab) is a humanized bispecific antibody which binds to factors IX-activated and X, speeding up the activation of factor X. It has bypassed some unmet needs in hemophilia A (HA) treatment, such as regimen (once weekly up to once monthly...
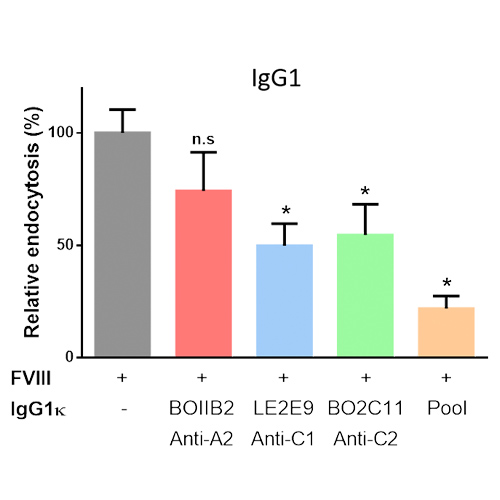
Nature of FVIII-containing immune complexes and induction of immune tolerance in patients with hemophilia A
Background and Aims 'Immune tolerance induction' (ITI) by repeated injections of high-dose factor VIII (FVIII) is the only strategy to eradicate inhibitory anti-FVIII IgG in hemophilia A patients.1,2 The epitope specificity and subclass of anti-FVIII IgG at initiation...
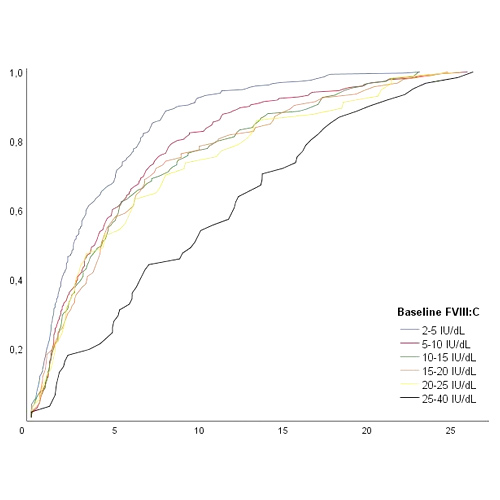
The timing of initial exposures to FVIII treatment in nonsevere hemophilia A
Introduction Although the majority of patients with hemophilia A have a moderate or mild factor VIII (FVIII) deficiency, there is a lack of detailed information on their treatment history. Data on the treatment history of nonsevere hemophilia A could provide important...
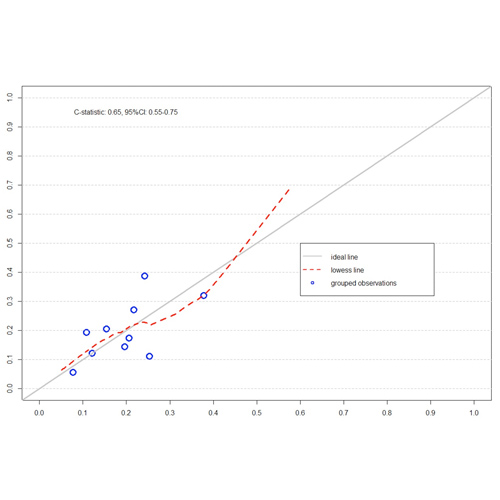
Performance of a clinical risk prediction model for inhibitor formation in severe hemophilia
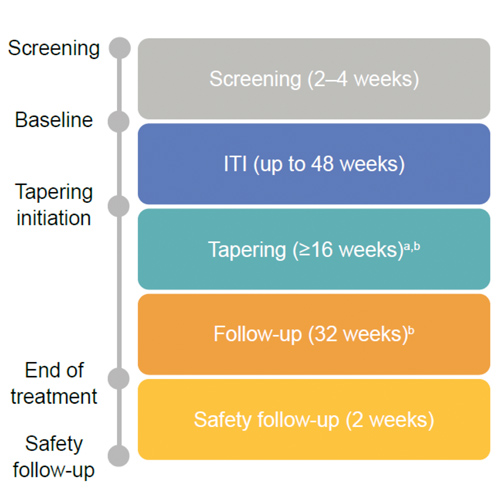
rFVIIIFc for first-time immune tolerance induction (ITI) therapy: interim results from the global, prospective verITI-8 study
Background Development of inhibitors to factor VIII (FVIII) treatment remains a major challenge in the treatment of haemophilia A1 Immune tolerance induction (ITI) therapy is the standard of care for inhibitor eradication2 ITI success rates of 50%–80% are reported2–4...
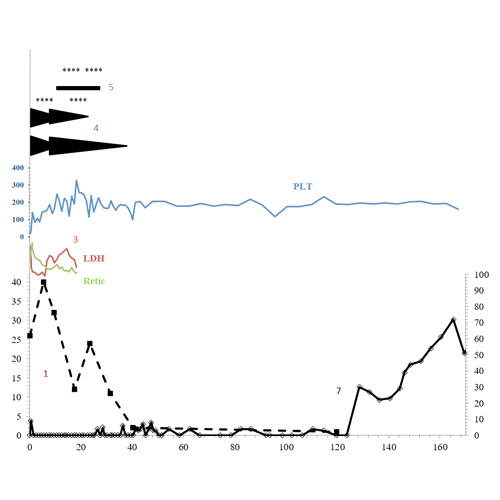
Successful treatment and durable remission of refractory thrombotic thrombocytopenic purpura (TTP) with belimumab
Introduction: We present a case of refractory TTP (thrombotic thrombocytopenic purpura) that ultimately recovered ADAMTS13 activity following treatment with belimumab. To our knowledge, this is the first report of this agent being used in TTP.Case Description: A 14...
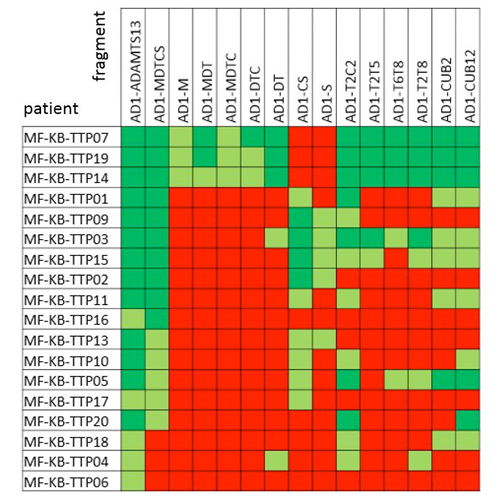
In-depth epitope mapping of anti-ADAMTS13 autoantibodies in immune-mediated thrombotic thrombocytopenic purpura patients using a large library of ADAMTS13 fragments
Background/Aims In immune mediated thrombotic thrombocytopenic purpura (iTTP), patients develop an immune response against the multidomain enzyme ADAMTS13. ADAMTS13 consists of a metalloprotease (M), and disintegrin-like (D) domain, eight thrombospondin type 1 repeats...
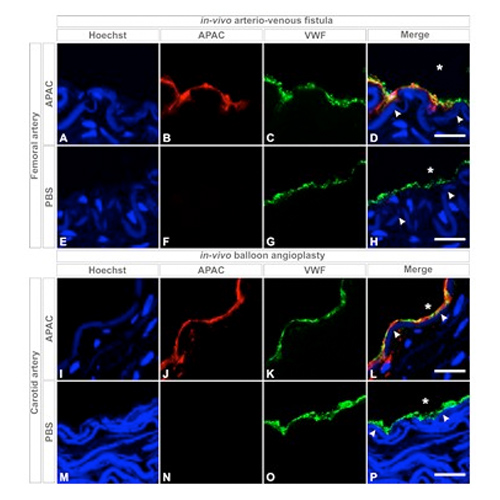
APAC, a dual AntiPlatelet and AntiCoagulant, towards a local vascular targeting antithrombotic
INTRODUCTION Mast cell heparin proteoglycans (HEP-PG) inhibit collagen- and von Willebrand factor (VWF)-mediated platelet thrombosis maintaining platelet adhesion1-4. APAC, platelet and fibrin inhibiting HEP-PG mimic1,5-7, is developed for antithrombotic management of...
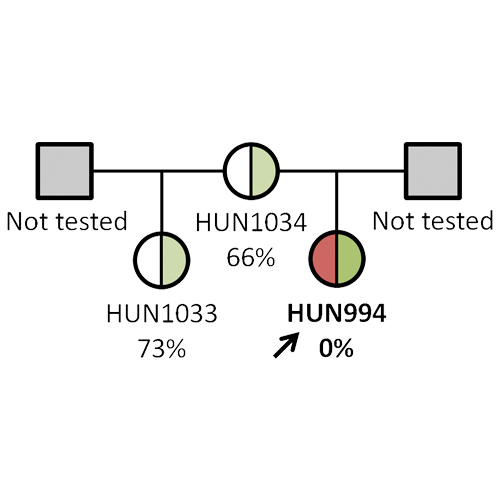
Investigation of the ADAMTS13 c.3178C>T mutation in hereditary and acquired thrombotic thrombocytopenic purpura
Introduction Thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy, characterized by the severe deficiency of ADAMTS13, which is responsible for cleaving ultra-large von Willebrand factor multimers. ADAMTS13 deficiency is caused by anti-ADAMTS13...
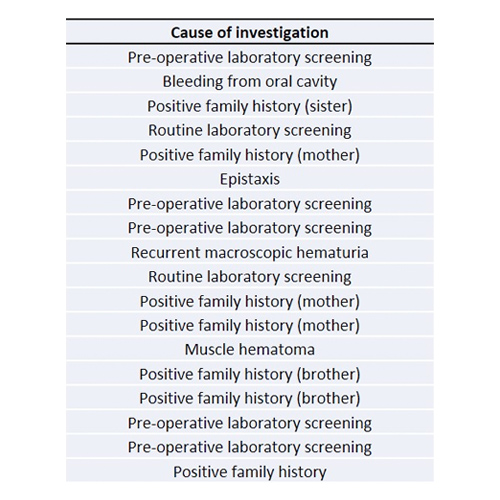
Rare bleeding disorders in the pediatric population of Northern Greece: a 10-year single center experience
Background: Rare bleeding disorders (RBDs) represent approximately 3–5% of all inherited coagulation deficiencies and are usually transmitted as autosomal recessive traits. Τheir clinical phenotype is characterized by considerable heterogeneity.Aim: To report on the...