A. Abdi1, F.R. Kloosterman1, C.L. Eckhardt1, C. Male2, G. Castaman3, K. Fischer4, E.A.M. Beckers5, M.J.H.A. Kruip6, K. Peerlinck7, M.E. Mancuso8, C. Santoro9, C.R. Hay10, H. Platokouki11, J.G. van der Bom12, S.C. Gouw1, D.P. Hart13, K. Fijnvandraat1, INSIGHT study group
1Amsterdam UMC, University of Amsterdam, Department of Pediatric Hematology, Immunology and Infectious Diseases, Emma Children’s Hospital, Amsterdam, the Netherlands; 2Medical University of Vienna, Department of Pediatrics, Vienna, Austria; 3Careggi University Hospital, Center for Bleeding Disorders, Department of Oncology, Florence, Italy; 4Van Creveldkliniek, University Medical Center Utrecht, Utrecht, the Netherlands; 5Maastricht University Medical Center, Department of Hematology, Maastricht, the Netherlands; 6Erasmus University Medical Centre, Department of Hematology, Rotterdam, the Netherlands; 7UZ Leuven, Division of Cardiovascular Disorders, Haemophilia Center, Leuven, Belgium; 8Fondazione Arianna IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Haemophilia and Thrombosis Center, Milan, Italy; 9Umberto I University Hospital of Rome, Cellular Biotechnology and Haematology Department, Rome, Italy; 10Manchester Royal Infirmary, Manchester University, Department of Hematology, Manchester, United Kingdom; 11Aghia Sophia Children’s Hospital, Haemophilia Centre-Haemostasis Unit, Athens, Greece; 12Leiden University Medical Center, Department of Clinical Epidemiology, Leiden, the Netherlands; 13Royal London Haemophilia Centre, Barts and the London School of Medicine and Dentistry, London, United Kingdom
Introduction
- Although the majority of patients with hemophilia A have a moderate or mild factor VIII (FVIII) deficiency, there is a lack of detailed information on their treatment history.
- Data on the treatment history of nonsevere hemophilia A could provide important information about the expected bleeding phenotype in patients with severe hemophilia A that are treated with non-replacement therapies.
Aim: to assess the timing of inital exposures to FVIII treatment in patients with nonsevere hemophilia A.
Methods
- We included patients born after 1980 with nonsevere hemophilia A from the INSIGHT cohort – a source population of patients with nonsevere hemophilia A (factor VIII activity 2-40 IU/dL) that received at least one exposure to FVIII concentrate between 1980 and 2011 in one of the 34 participating hemophilia treatment centers.
- Data on the first exposure to FVIII treatment were available for all patients included in this study.
- Detailed information on the timing of all FVIII exposure days during study follow-up, was available for a subgroup of patients.
- The inclusion of patients is presented in Figure 1.
Patient inclusion
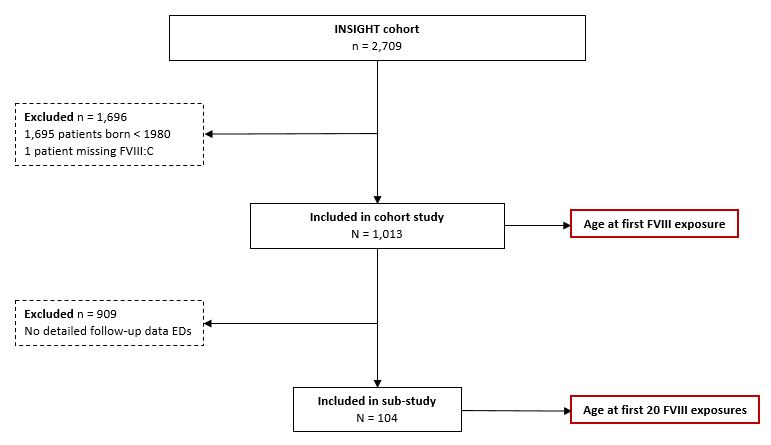
Figure 1. Flowchart of patients included in the study.
Timing of initial exposures to FVIII treatment
Cohort
- Within the total study cohort (n = 1013), there were 356 (35%) patients with moderate hemophilia A and 657 (65%) with mild hemophilia A.
- The median baseline FVIII level was 8 IU/dL (IQR 4-15). The age at the end of study follow-up was 16 years (IQR 10-23).
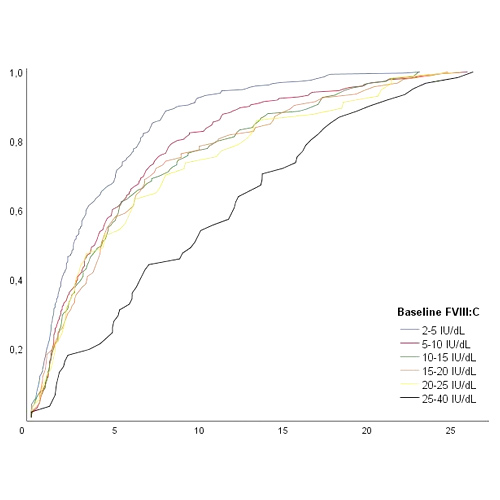
- The median age at the first exposure to FVIII treatment was 2.5 years (IQR 1.2-5.7) for patients with a FVIII level of 2-5 IU/dl, increasing to 9.7 years (IQR 4.8-16.0) for patients with a FVIII level of 25-40 IU/dL. (Figure 2)
Sub-study
- A total of 2824 FVIII exposure days were recorded in 104 patients in the sub-study.
- The median baseline FVIII level was 6 IU/dL (IQR 4-11).
- The median age at the first 20 exposures to FVIII treatment is presented in Figure 3.
Age at first FVIII exposure
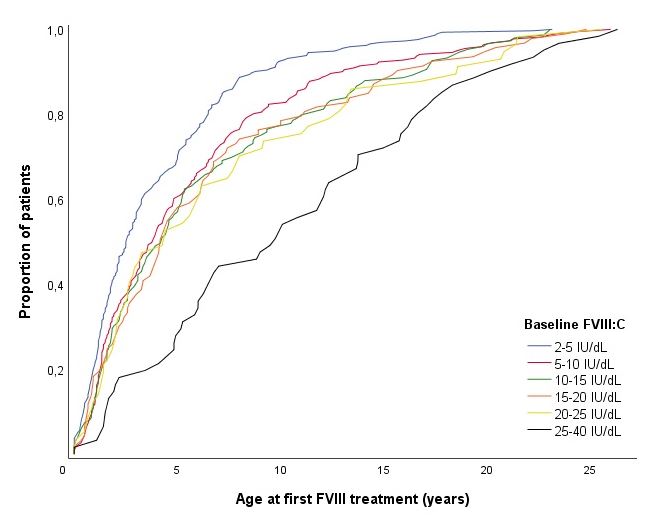
Figure 2. Age at first FVIII exposure in the total cohort (n = 1013) stratified for baseline FVIII level.
Age at first 20 FVIII exposures
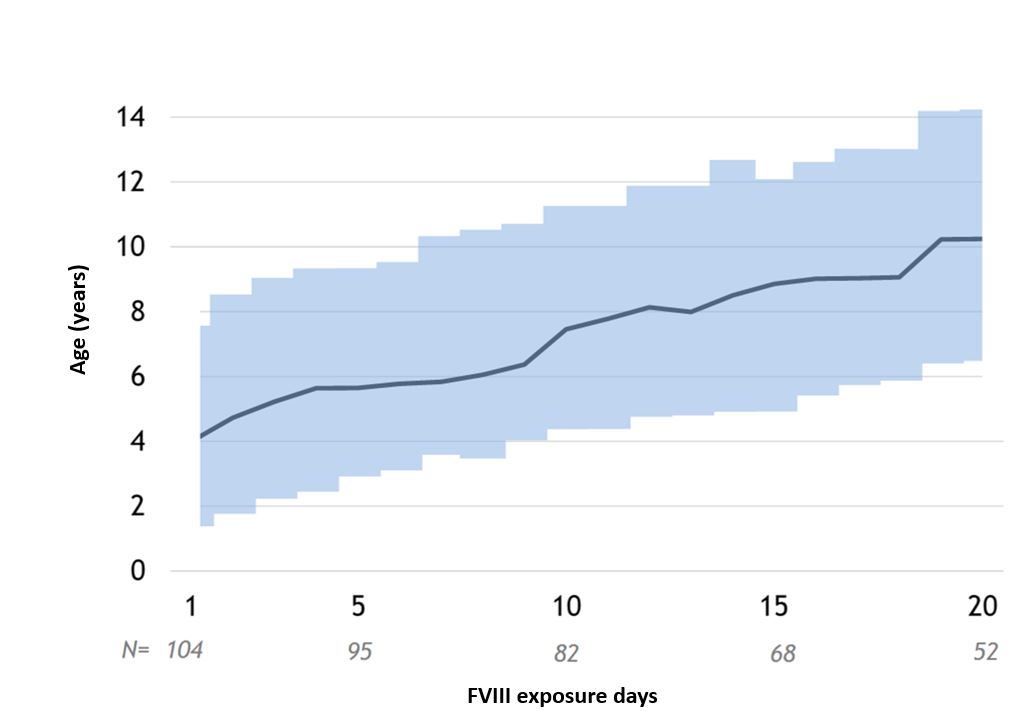
Figure 3. Age at the first 20 FVIII exposures in the sub-study (n = 104). Dark line is the median, shaded area the IQR.
Conclusion
- The timing of the first exposure to FVIII treatment in nonsevere hemophilia A ranges from 2.5 to 9.7 years, depending on the baseline FVIII level.
- Our data may provide insight in the expected effect of non-replacement therapies in patients with severe hemophilia A, suggesting a considerable postponement in the timing of initial FVIII treatment.