Kadri Kangro1, Elien Roose1, An-Sofie Schelpe1, Edwige Tellier2, Gilles Kaplanski2, Jan Voorberg3, Simon F. De Meyer1, Andres Männik4, Karen Vanhoorelbeke1
1Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium; 2INSERM, Aix-Marseille University, Marseille, France; 3Department of Molecular and Cellular Hemostasis, Sanquin-Academic Medical Center Landsteiner Laboratory, Amsterdam, The Netherlands; 4Icosagen Cell Factory, Õssu, Kambja, Estonia
Background/Aims
- In immune mediated thrombotic thrombocytopenic purpura (iTTP), patients develop an immune response against the multidomain enzyme ADAMTS13. ADAMTS13 consists of a metalloprotease (M), and disintegrin-like (D) domain, eight thrombospondin type 1 repeats (T1-T8), cysteine-rich (C), spacer (S), and 2 CUB domains (CUB12).
- Epitope mapping using relatively large fragments of ADAMTS13 revealed that almost all iTTP patients have anti-CS antibodies while up to 60% of the patients also have autoantibodies against other ADAMTS13 domains.
- In this project we aimed at developing a large library of ADAMTS13 fragments to profile the anti-ADAMTS13 autoantibodies in iTTP patients.
Materials/Methods
- A library of 16 ADAMTS13 fragments, comprising individual or multiple domains of ADAMTS13: M, MDT, MDTC, DTC, DT, CS, C, S, T2T5, T6T8, T2T8, CUB1, CUB2, CUB12, MDTCS, T2C2, and ADAMTS13 was created (Figure 1).
- All ADAMTS13 fragments were expressed as a fusion protein with albumin domain 1 (AD1) in Chinese hamster ovary cells, and purified using affinity chromatography (Figure 2).
- The correct folding of the fragments was tested using 18 anti-ADAMTS13 monoclonal antibodies (mAbs) with known epitopes using ELISA (Figure 3).
- The anti-ADAMTS13 autoantibody profile of 18 iTTP patients was fine mapped using this large library of ADAMTS13 fragments (Figure 4).
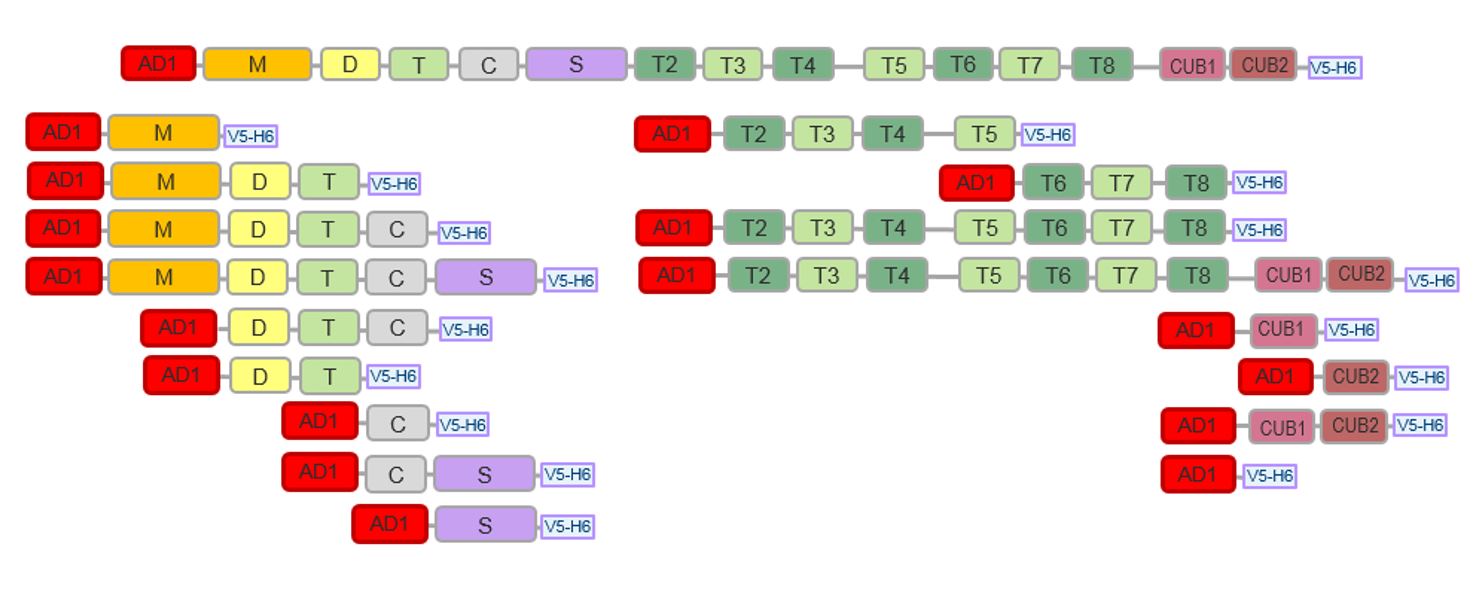
Figure 1. Schematic domain representation of AD1-ADAMTS13 constructs
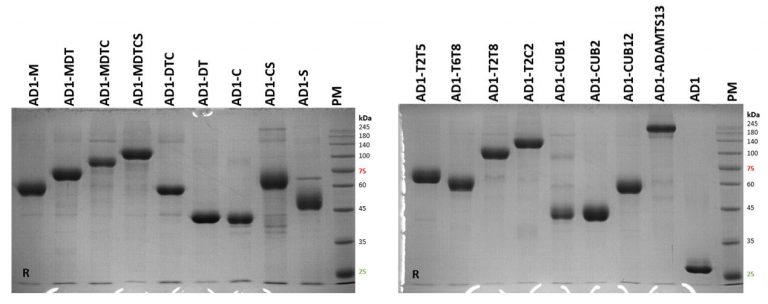
Figure 2. SDS-PAGE analysis of purified AD1-ADAMTS13 fragments
Results
- Of the 16 AD1-ADAMTS13 fragments, only the AD1-C, AD1-S and AD1-CUB1 fragments were not correctly folded as identified in ELISA using the 18 mAbs (Figure 3).
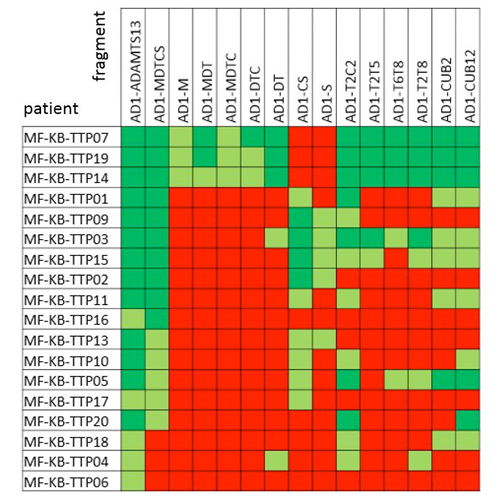
- In-depth epitope mapping of iTTP patient samples using this collection of ADAMTS13 fragments (Figure 4, Table 1), showed the antibody profile of
- 83.3% anti-MDTCS
- 16.7% anti-M
- 27.8% anti-DT
- 55.6% anti-CS
- 72.2% anti-T2C2
- 27.8% anti-T2T5
- 27.8% anti-T6T8
- 61.1% anti-CUB12
- 83.3% anti-MDTCS

Table 1. Epitope mapping of anti-ADAMTS13 antibodies for 18 iTTP patients
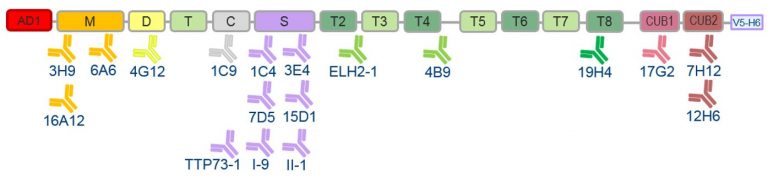
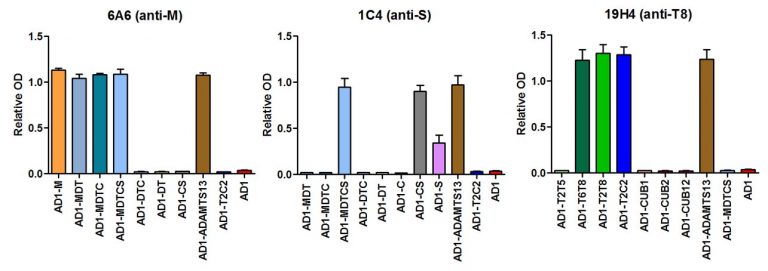
Figure 3. Binding of anti-ADAMTS13 monoclonal antibodies with known epitopes to AD1-ADAMTS13 fragments
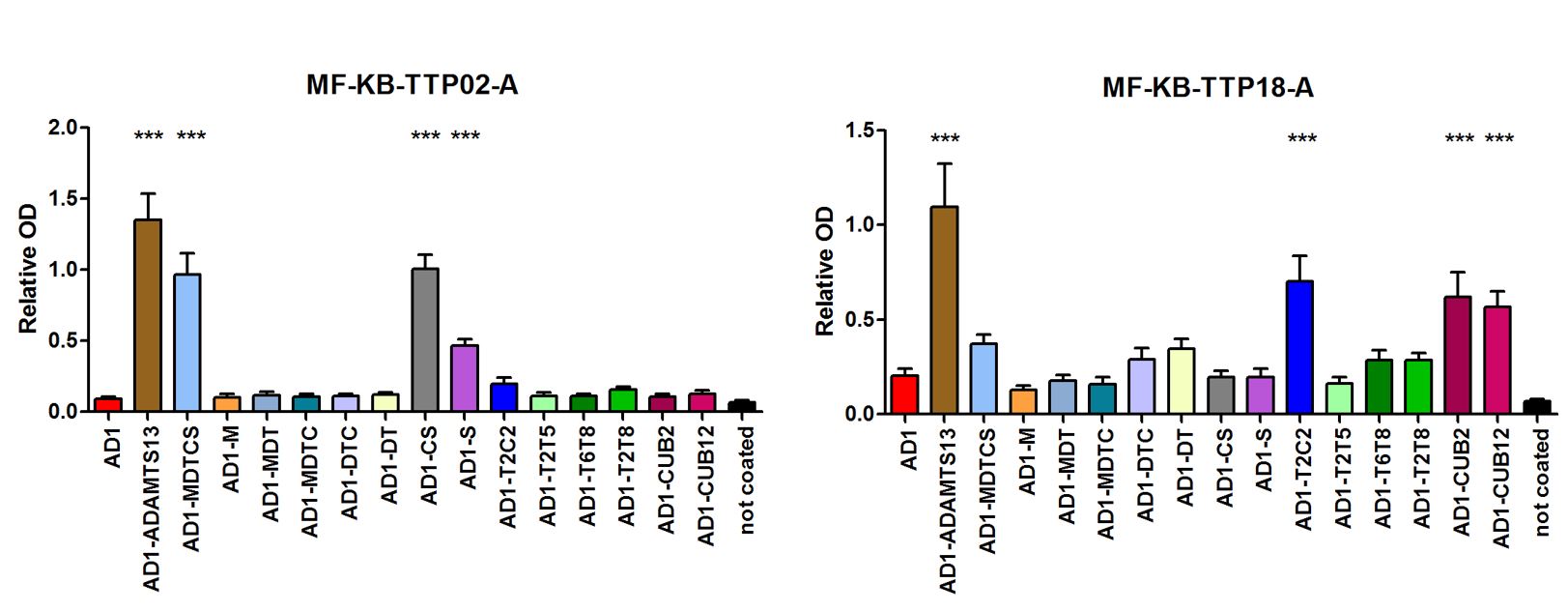
Figure 4. Binding of anti-ADAMTS13 antibodies from iTTP patient plasma to AD1-ADAMTS13 fragments
Conclusion
- We have developed a powerful tool to profile the iTTP patients according to their anti-ADAMTS13 antibodies.
- In-depth epitope mapping of the anti-ADAMTS13 autoantibodies allows to get a better insight in the immune response in iTTP patients.

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the
Marie Skłodowska-Curie grant agreement No 675746