Mélissa Bou Jaoudeh, Victoria Daventure, Sandrine Delignat, Angelina Mimoun, Sébastien Lacroix-Desmazes
Centre de Recherche des Cordeliers, INSERM, Sorbonne Université, USPC, Université Paris Descartes, Université Paris Diderot, F-75006 Paris, France INSERM, Unité Mixte de Recherche Scientifique 1176, Université Paris-Sud, Université Paris-Saclay, Le Kremlin-Bicêtre, France
Background and Aims
‘Immune tolerance induction’ (ITI) by repeated injections of high-dose factor VIII (FVIII) is the only strategy to eradicate inhibitory anti-FVIII IgG in hemophilia A patients.1,2 The epitope specificity and subclass of anti-FVIII IgG at initiation of ITI were proposed as predictors of ITI outcome.3 FVIII injection to patients with circulating anti-FVIII IgG leads to the formation of FVIII-containing immune complexes (FVIII-IC).4 We hypothesize that the nature of the FVIII-IC that form at the start of ITI dictates ITI outcome.
Material and Methods
We produced the inhibitory human monoclonal anti-A2 (BOIIB2), anti-C1 (LE2E9) and anti-C2 (BO2C11) IgG in IgG1к and IgG4к formats. We validated the Fab and Fc portions of IgG by assessing their capacity to bind to FVIII and different FcR, inhibit VWF-binding to FVIII and neutralize FVIII pro-coagulant activity using ELISA, Bethesda assay and SPR. Then, the formation of FVIII-IC upon incubation of FVIII with anti-FVIII IgG was validated after immunoprecipitation. The effect of the composition of FVIII-IC (domain specificity, mono/oligoclonality, subclass) on FVIII endocytosis by monocyte-derived dendritic cells (MODC) was investigated.5
Results
Validation and characterization of the recombinant IgG
Validation of IgG1 and IgG4 Fab portions involved testing their capacity to interact with FVIII in an indirect ELISA. BOIIB2, BO2C11 and LE2E9 bound to FVIII with no difference between IgG1κ and IgG4κ subclasses (Figure 1).
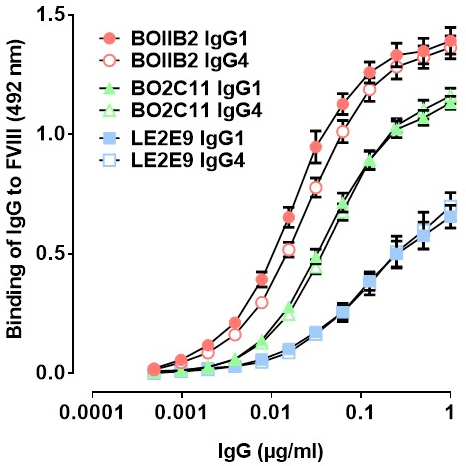
Figure 1 | Binding of human monoclonal recombinant anti-FVIII IgG1κ and IgG4κ to FVIII. Plates were coated with FVIII (Advate, 2.5 μg/mL) and incubated with serial dilutions of BO2C11 (green triangles), BOIIB2 (red circles) or LE2E9 (blue squares) IgG1κ (full symbols) or IgG4κ (empty symbols) subclass. Bound IgG were detected by addition of an HRP-labeled mouse monoclonal Ab specific for human Fcγ and OPD substrate. Binding intensities are expressed in arbitrary units (AU, mean ± SD from 3 independent experiments) of optical density measured at 492 nm.
Formation of FVIII-IC in vitro
The formation of FVIII-IC upon incubation of FVIII with a 5-molar excess of anti-FVIII IgG, was validated after immunoprecipitation using protein G-coated Agarose beads, in the case of BO2C11. Both BO2C11 IgG1к and IgG4к formed IC with FVIII (Figure 2).
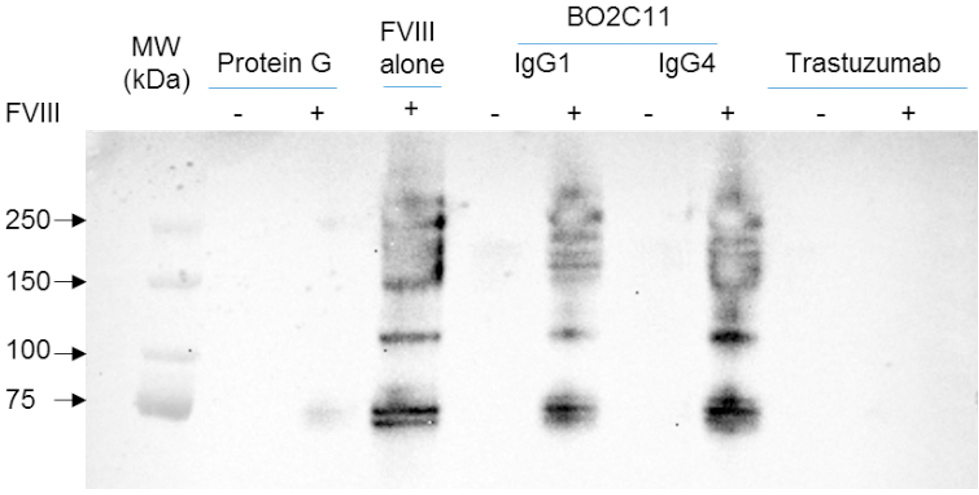
Figure 2 | Generation and detection of FVIII-immune complexes. Eluates obtained by immunoprecipitation were collected and analyzed by Western blot. FVIII-IC were identified by revelation with a biotinylated sheep anti-human FVIII antibody (SAF8C) under non-reducing conditions. Non eluted FVIII (FVIII alone) was used as a positive control. Trastuzumab, a monoclonal Ab that doesn’t recognize FVIII, was used as a negative control as well as eluate of protein G alone or incubated with FVIII. Molecular weight markers (MW) are shown on the left lane of the blot.
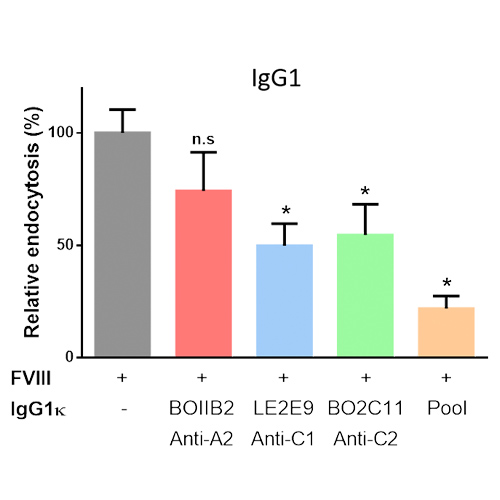
Endocytosis of FVIII-IC by MODC
The incubation of FVIII complexed to anti-FVIII IgG resulted in a significant decrease in FVIII uptake by MODC, as compared to FVIII incubated with cells alone. The decrease was more prominent for BO2C11 and LE2E9 IgG, than for BOIIB2 (Figure 3). It was not affected by the change in IgG subclass (Data not shown).
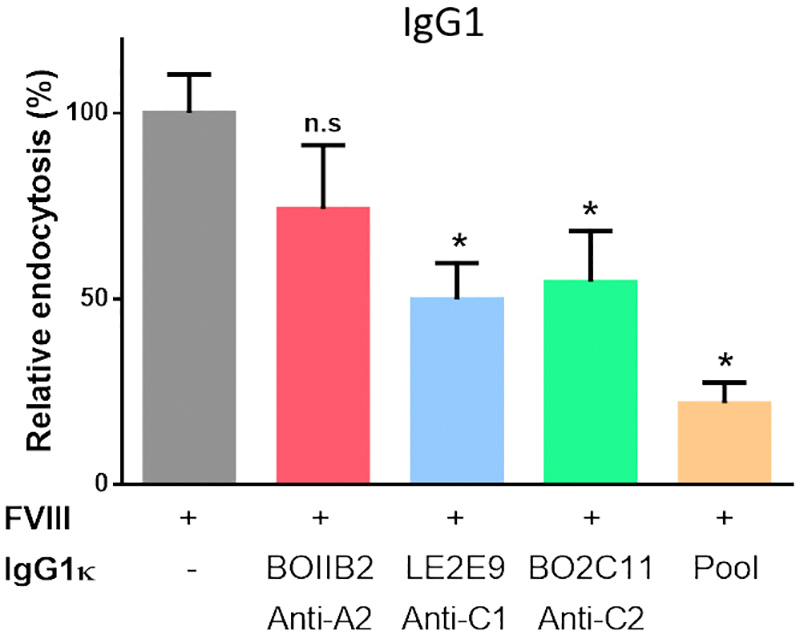
Figure 3 | In vitro FVIII-IC uptake by immature MODC. FVIII-FITC (20 nM) was pre-incubated alone or with 5 molar excess of single IgG1к (BO2C11, BOIIB2, LE2E9) or with a pool of the three IgG1к. Uptake by MODC was analyzed by flow cytometry. Results are expressed as the percentage of the difference of median fluorescence intensity measured at 37°C and 4°C (ΔMFI (37°C – 4°C)), whereby 100% corresponds to ΔMFI obtained with FVIII-FITC alone. Graphs are representative of 2 different donors. Statistical significance was assessed using the non-parametric 2-sided Mann-Whitney test (n.s: non-significant; *: p value < 0.05).
Conclusion
The present work validates the production and functions of human recombinant anti-FVIII antibodies (Abs) in IgG1k and IgG4k formats. The change in IgG subclass was not associated with drastic alteration of IgG functions. The formation of FVIII-IC reduces FVIII endocytosis by MODC, particularly if the IC contains anti-C1 and/or anti-C2 Abs. Future work includes studying the repercussion of the nature of FVIII-IC on FVIII uptake by other types of professional antigen-presenting cells (APCs) including blood DCs, macrophages and B cells, on their maturation and capacity to polarize CD4+ T-cell responses. At term, our findings will foster the rational design of FVIII-IC with tolerogenic properties for the treatment of inhibitor-positive patients.
REFERENCES
- Ehrenforth, S. et al. (1992) The Lancet 339, 594–598.
- Ljung, R. et al. (2019) Eur. J. Haematol. 102, 111–122.
- Minno, G. D. (2014) Haemophilia 20, 27–43.
- Kazatchkine, M. D. (1980) Clin Exp Immunol 39, 315–320.
- Hartholt, R.B et al. (2017) J. Thromb. Haemost. 15, 329–340.