R. Camelo1, N. Dantas-Silva2, J. Alvares-Teodoro3
1Universidade Federal de Minas Gerais, Faculty of Medicine, Belo Horizonte, Brazil; 2Fundação HEMOMINAS, Belo Horizonte, Brazil; 3Universidade Federal de Minas Gerais, Faculty of Pharmacy, Belo Horizonte, Brazil

BACKGROUND
Emicizumab (MC-Ab) is a humanized bispecific antibody which binds to factors IX-activated and X, speeding up the activation of factor X. It has bypassed some unmet needs in hemophilia A (HA) treatment, such as regimen (once weekly up to once monthly infusion) and route of administration (subcutaneous). Although it is an effective non-replacement alternative in the prophylaxis of people with HA (PwHA) with (PwHAi) or without inhibitor, its safety has not been clarified yet, and a few cases of thrombosis and development of anti-MC-Ab antibody have been described.
The aim of this project is to create a national registry to follow up PwHA receiving MC-Ab.
METHODS
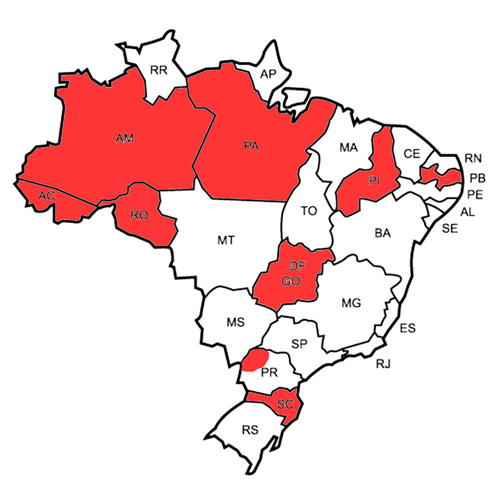
EMCase is an observational study and any PwHA receiving MC-Ab can be included. The treatment will be decided among the patient, the physician and the interdisciplinary team of the hemophilia treatment center (HTC) of each Brazilian state (Figure). The research group will develop a brochure with suggestions on classical outcome assessment tools (e.g., bleeding rate, joint health, absenteeism, adherence, quality of life and mortality) which can be evaluated as the judgement of the HTC team. Outcome data, laboratory results and therapeutic progression will be compiled yearly over 10 years. Pharmacovigilance and economic analyses will also be included. Finally, a national guidance will be developed.
RESULTS
In Brazil, MC-Ab was approved in 2018 only for treatment of PwHAi*. In 2015, there were 250 patients on immune tolerance and the failure rate of this treatment has been described about 20%. Consequently, we expect to register at least 50 PwHAi.
*MC-Ab was approved for PwHA after the acceptance of this abstract.
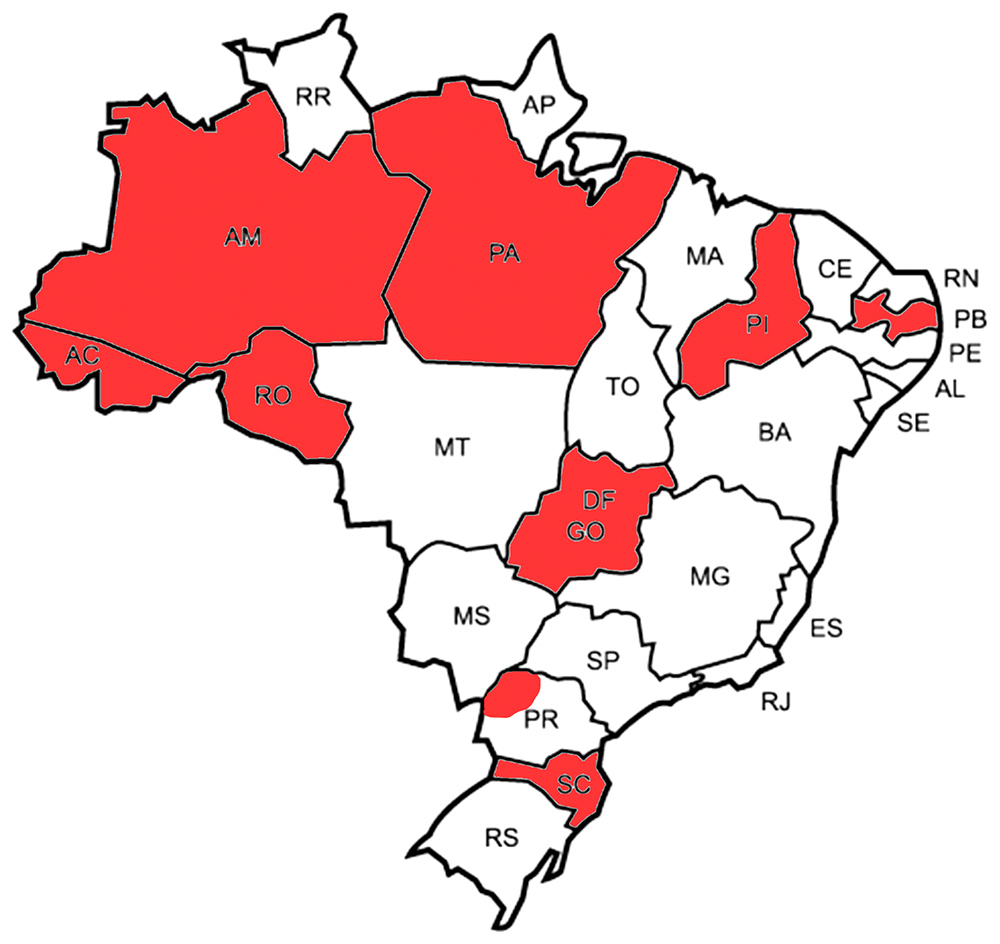
Figure. Brazilian states which are already participating in the study (in red).
CONCLUSION
We expect to establish some outcome assessment tools and laboratory tests to aid the interdisciplinary team to manage hemophilia treatment with MC-Ab as well as to help to clarify the safety of this bispecific antibody.
FINANCIAL SUPPORT
There was not financial support for this research.
DISCLOSURES
There was not conflict of interest for this research.
REFERENCES
Alzoebie A, Belhani M, Eshghi P, Kupesiz AO, Ozelo M, Pompa MT, Potgieter J, Smith M. Establishing a harmonized haemophilia registry for countries with developing health care systems. Haemophilia. 2013;19:668-73.
Berntorp E. Future of haemophilia outcome assessment: registries are key to optimized treatment. J Intern Med. 2016;279:498-501.
Dolan G, Makris M, Bolton-Maggs PH, Rowell JA. Enhancing haemophilia care through registries. Haemophilia. 2014;20(Suppl4):121-9.
Ferreira AA, Leite IC, Bustamante-Teixeira MT, Guerra MR. Hemophilia A in Brazil – epidemiology and treatment developments. J Blood Med. 2014 Sep 23;5:175-84.
Fischer K, Poonnoose P, Dunn AL, Babyn P, Manco-Johnson MJ, David JA, van der Net J, Feldman B, Berger K, Carcao M, de Kleijn P, Silva M, Hilliard P, Doria A, Srivastava A, Blanchette V; participants of the International Symposium on Outcome Measures in Hemophilic Arthropathy. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia. 2017;23:11-24.
Keipert C, van den Berg HM, Keller-Stanislawski B, Hilger A. Haemophilia registries to complement clinical trial data: a pious hope or an urgent necessity?: Reflections on a possible way forward. Haemophilia. 2016;22:647-50.
Kitazawa T, Shima M. Emicizumab, a humanized bispecific antibody to coagulation factors IXa and X with a factor VIIIa-cofactor activity. Int J Hematol. 2018 (ahead of print).
Langer AL, Etra A, Aledort L. Evaluating the safety of emicizumab in patients with hemophilia A. Expert Opin Drug Saf. 2018;17:1233-7.
Mahlangu J, Oldenburg J, Paz-Priel I, Negrier C, Niggli M, Mancuso ME, Schmitt C, Jiménez-Yuste V, Kempton C, Dhalluin C, Callaghan MU, Bujan W, Shima M, Adamkewicz JI, Asikanius E, Levy GG, Kruse-Jarres R. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018;379:811-22.
Mahlangu JN. Bispecific Antibody Emicizumab for Haemophilia A: A Breakthrough for Patients with Inhibitors. BioDrugs. 2018;32:561-70.
Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, Santagostino E, Kruse‑Jarres R, Negrier C, Kessler C, Valente N, Asikanius E, Levy GG, Windyga J, Shima M. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809-18.
Osooli M, Berntorp E. Inhibitors in haemophilia: what have we learned from registries? A systematic review. J Intern Med. 2015;277:1-15.
Rezende SM, Pinheiro K, Caram C, Genovez G, Barca D. Registry of inherited coagulopathies in Brazil: first report. Haemophilia. 2009;15:142-9.
Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, Fukazawa N, Yoneyama K, Yoshida H, Nogami K. Factor VIII-Mimetic Function of Humanized Bispecific Antibody in Hemophilia A. N Engl J Med. 2016;374:2044-53.
Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, Kasai R, Yoneyama K, Yoshida H, Nogami K. Long-term safety and efficacy of emicizumab in a phase 1/2 study in patients with hemophilia A with or without inhibitors. Blood Adv. 2017;1:1891-9.
Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, Shima M. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. 2016;127:1633-41.
Young G, Callaghan M, Dunn A, Kruse-Jarres R, Pipe S. Emicizumab for hemophilia A with factor VIII inhibitors. Expert Rev Hematol. 2018;11:835-46.