Ryan R Woods MD*12, Andrew M. Farland MD12, Peter J Miller MD1, John Owen MD1, Kathy Batt MD1, Lauren Imboden PA-C, Stephanie L. Bollinger RN1, Melanie Kiser2, Caryl Adams2, Becky Combs2, Diane Meares2
1Wake Forest Baptist Medical Center and Wake Forest School of Medicine, Department of Internal Medicine, Section on Hematology and Oncology; 2Special Hematology Laboratory, Departiemnt of Internal Medicine. Winston-Salem, North Carolina USA
Introduction:
We present a case of refractory TTP (thrombotic thrombocytopenic purpura) that ultimately recovered ADAMTS13 activity following treatment with belimumab. To our knowledge, this is the first report of this agent being used in TTP.
Case Description:
A 14 year old male presented to a local ER with left facial numbness, bilateral arm paresthesia and weakness.
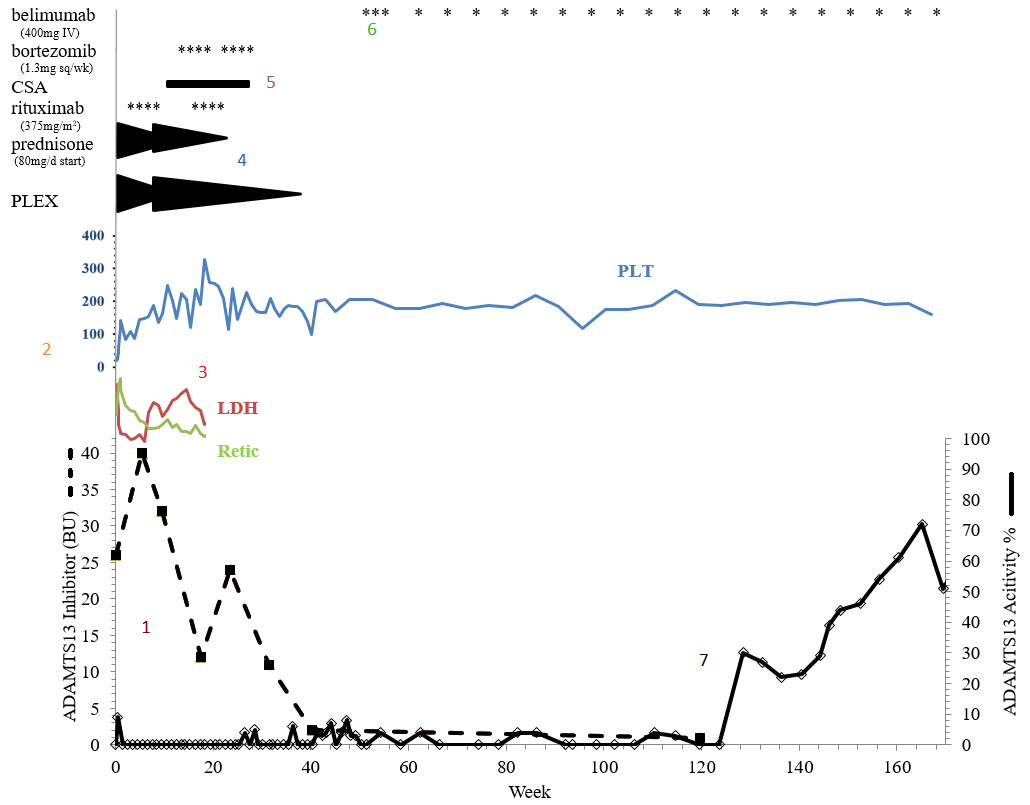
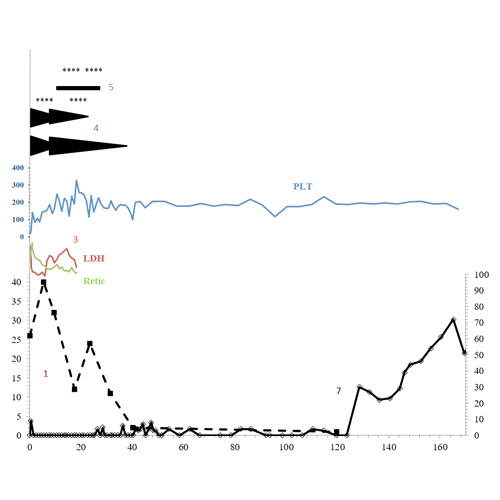
1. On initial laboratory examination, he was found to have thrombocytopenia (platelet count 13×103/uL) and evidence of microangiopathic hemolytic anemia (hemoglobin 10.5g/dL, undetectable haptoglobin, elevated lactate dehydrogenase, and over 20 schistocytes/HPF). TTP was suspected, and the patient was started on plasma exchange (PLEX), steroids, antiplatelet, and folic acid pending confirmatory ADAMTS13 activity. ADAMTS13 was undetectable (<3% activity of normal) with a 26 BU inhibitor at presentation.
2. Inhibitor titer increased to 40 BU during initial therapy. The patient required escalation of PLEX due to evidence of refractory hemolysis (anemia, LDH, and elevated retic).
3. The patient maintained a stable platelet count in platelet count and improvement in hemolytic markers by week 10, but maintained a high-titer inhibitor to ADAMTS13.
4. Per our institutional practice, a PLEX taper was recommended with adjustments to aphresis schedule and immunosuppression based upon pre-and post-pheresis ADAMTS13 activity trends.
5. Despite sequential immunosuppressive/immunomodulatory agents (including high-dose steroids, rituximab times two courses of four weekly doses, cyclosporine for 140 days, bortezomib times two monthly cycles) a 10 BU inhibitor remained at 36 weeks after presentation, with continued low ADAMTS13 activity level.
6. At 40 weeks, in an attempt to improve the durability of remission despite these low residual ADAMTS13 activity, the patient was started on belimumab 400 mg IV every two weeks times three doses followed by 400 mg monthly. A clinical remission was observed with ADAMTS13 activity between 3-5% for the next 80 weeks.
7. Disappearance of the inhibitor and continued improvement of ADAMTS13 activity level was noted at 124 weeks. The patient remains well on maintenance belimumab without relapse with ADAMTS13 over 70% of normal.
Discussion:
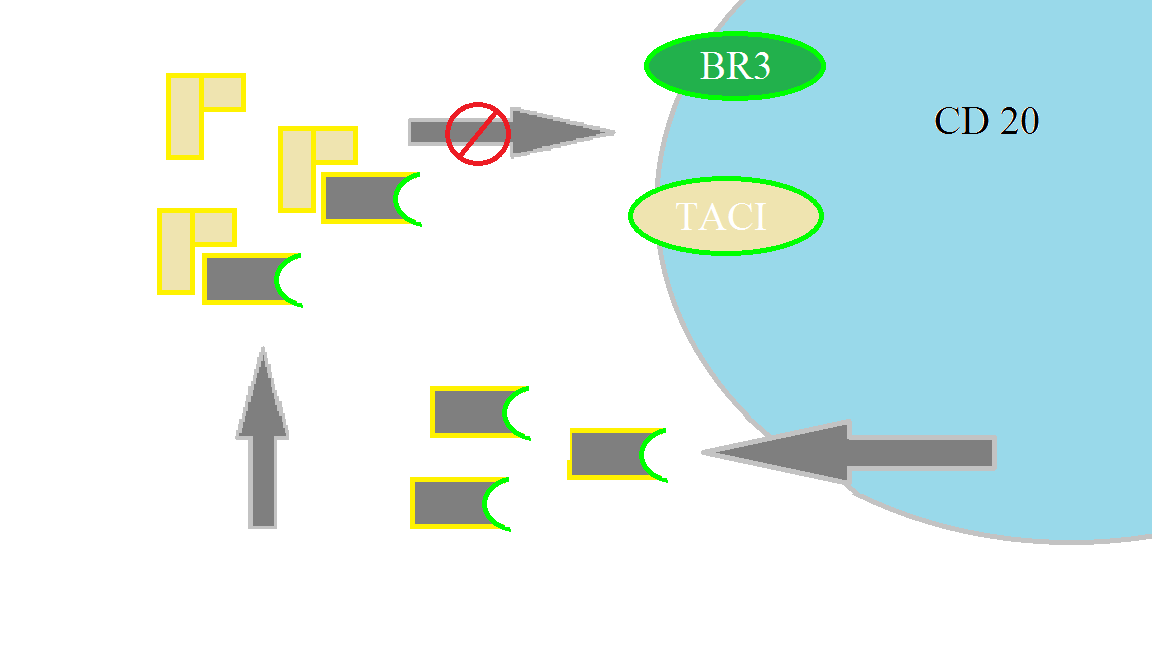
The anti B-cell activating factor agent belimumab has proven efficacy in autoimmune syndromes, and is FDA approved for the treatment of systemic lupus erythematosus.
B-lymphocyte stimulator (BLyS) cytokine is expressed by mature lymphoid cells. BLyS binds surface receptors on autoreactive B-cells, signaling them to survive, mature, and differentiate. Belimumab binds to soluble BLyS to downregulate reactive B cells, stalling antibody (or autoantibody) production.
In TTP, Belimumab represents a novel, but logical immunomodulatory/immunosuppressive agent, and proved effective in this case. Additional clinical investigation is warranted.