Joline L. Saes1,2, Saskia E.M. Schols1,2, Kathleen F. Betbadal3, Mark van Geffen4, Kitty Verbeek-Knobbe5, Sweta Gupta3, Brandon M. Hardesty3, Amy D. Shapiro3, Waander L. van Heerde2, 4
1Department of Hematology, Radboud university medical center, Nijmegen, The Netherlands; 2Haemophilia Treatment Center Nijmegen – Eindhoven – Maastricht, The Netherlands; 3Indiana Hemophilia & Thrombosis Center, Indianapolis, USA; 4Enzyre BV, Noviotech Campus, Nijmegen, The Netherlands; 5Laboratory for Hematology, Department of Laboratory Medicine, Radboud university medical center, Nijmegen, The Netherlands
Introduction:
Deficiencies of plasminogen and plasminogen activator inhibitor type 1 (PAI-1) are rare disorders of fibrinolysis. Conventional laboratory assays of fibrinolysis lack specificity and validation. Moreover, there is a weak correlation of the assays with clinical symptoms.
Methods:
The Nijmegen Hemostasis Assay (NHA) was used to simultaneously measure thrombin and plasmin generation in five patients with plasminogen deficiency and ten patients with complete PAI-1 deficiency. Parameters analysed were: lag-time ratio, thrombin peak time ratio, thrombin peak height, thrombin potential (AUC), fibrin lysis time, plasmin peak height and plasmin potential. All parameters were expressed as a percentage compared to a reference value of fifty-three healthy individuals.
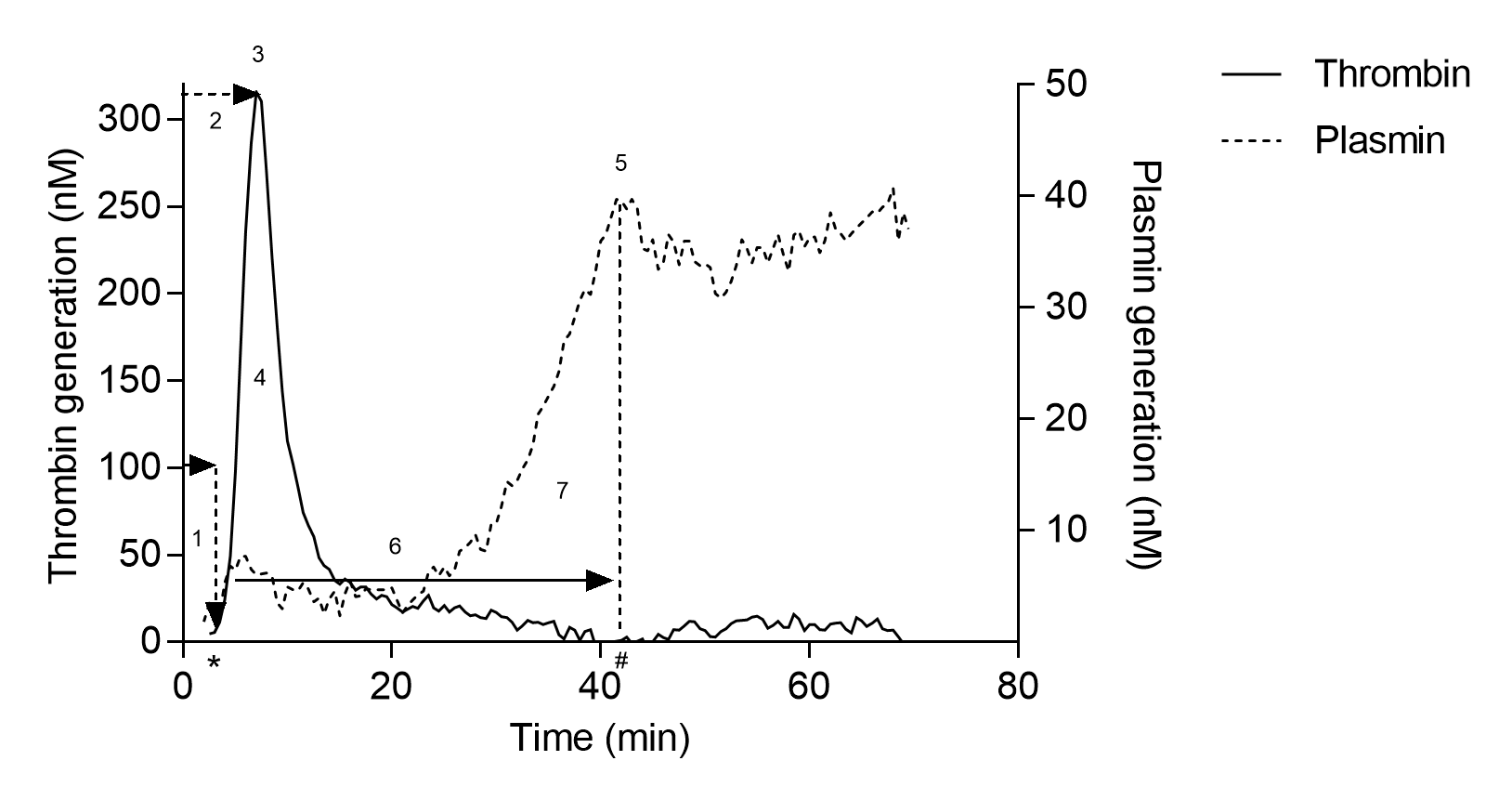
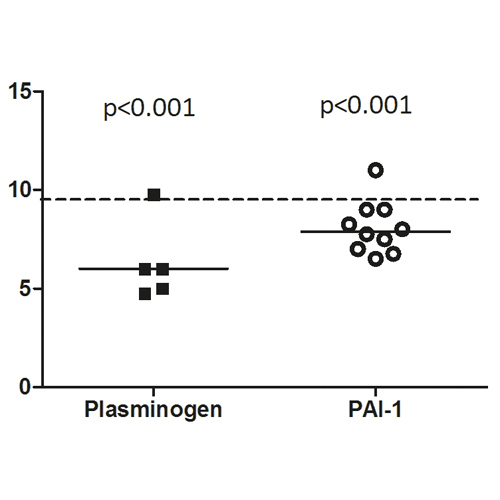
Figure 2
Results:
Five patients including 3 females (6, 7, 49 years) and 2 males (10, 37 years) with plasminogen deficiency were evaluated. Patients with PLGD had plasminogen activity levels ranging from 5 IU/dL to 23 IU/dL (normal level >51 IU/dL). Plasminogen antigen levels ranged from 12 mg/L to 64 mg/L (normal level >90 mg/L) consistent with type 1 deficiency. None of the PLGD patients experienced a thrombotic or haemorrhagic event. All patients had experienced ligneous conjunctivitis, and two patients had ligneous gingivitis as well. Samples were obtained without exposure to exogenous therapy.
The PAI-1 deficient patients had absent PAI-1 activity and antigen levels, inferred by a known genetic null mutation. Of the ten patients evaluated there were 7 females (ages 16-37 years), and 3 males (ages 16-35 years). PAI-1 deficient patients experienced bleeding manifestations of varying severity. Maenorrhagia was most prevalent, occurring in the majority of females (5 of 7); other symptoms included major bleeding such as intracranial haemorrhage after trauma, other bleeding events associated with injury, trauma or invasive procedures, antenatal and postpartum bleeding in gravid females, haemorrhagic rupture of ovarian cyst, and minor mucocutaneous bleeding.
Thrombin and plasmin generation curves are shown in the figure on the left.
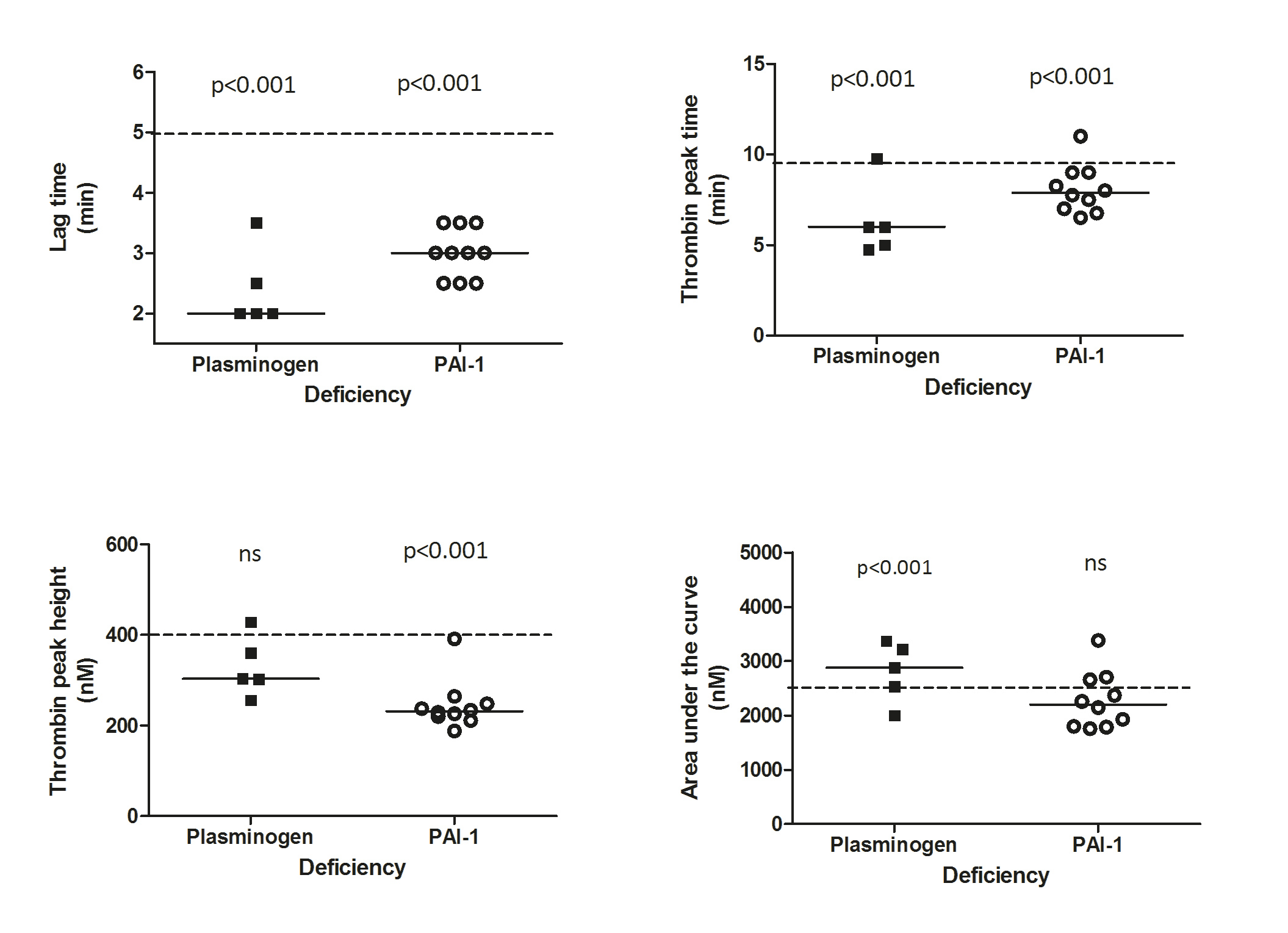
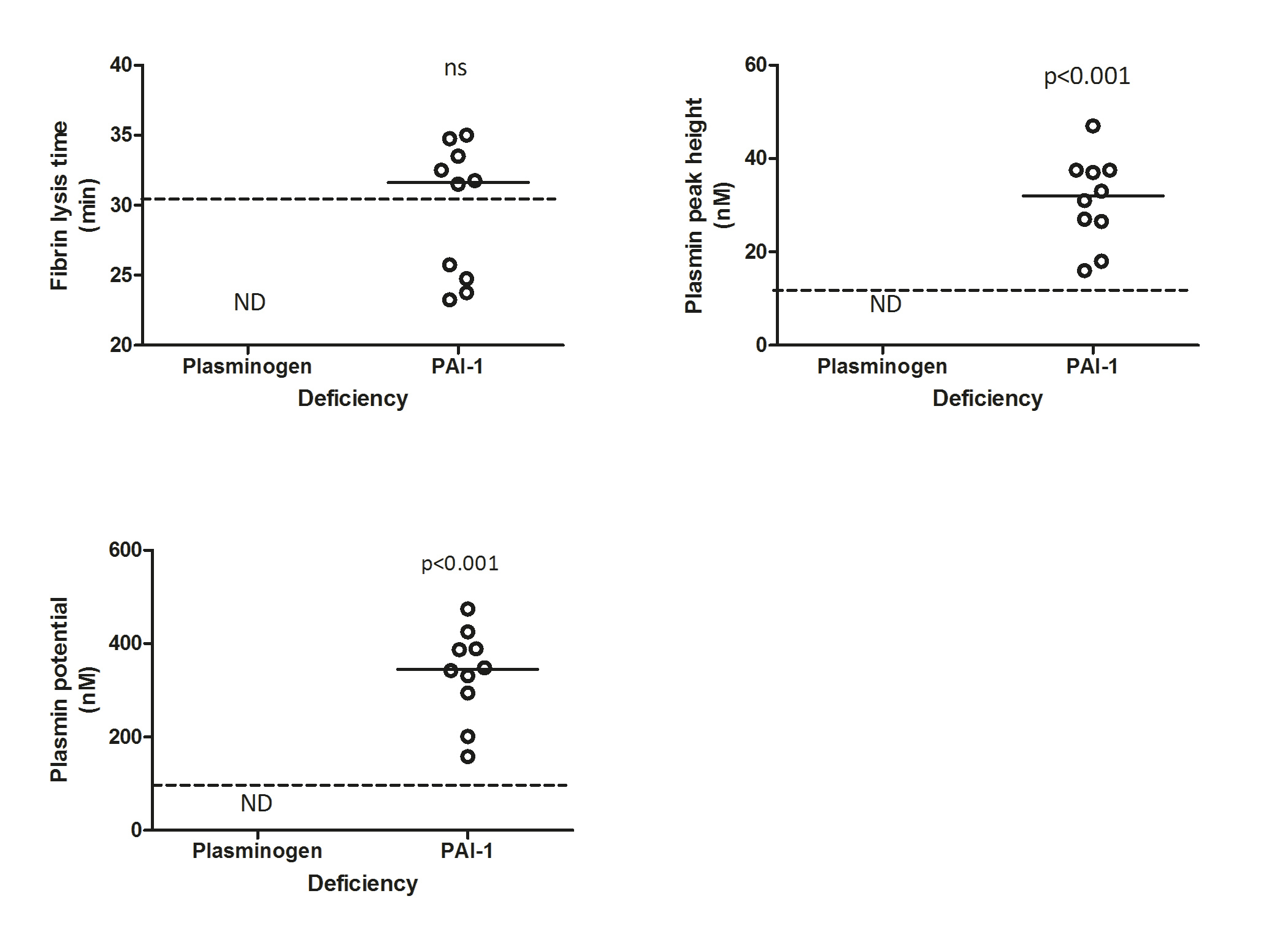
PLGD patients demonstrated a short initiation with a median lag-time of 38% (p<0.001, IQR 38-57%) and thrombin peak time of 59% (p<0.001, IQR 48-78%) as compared to the reference group of healthy controls. Propagation was relatively normal with a thrombin peak height of 87% (IQR 65-113%) with slightly enhanced AUC of 139% (p<0.001, IQR 109-159%). No measurable plasmin generation was observed in PLGD patients, except for patient 4, whose baseline plasminogen activity level was 23%.
When compared to healthy controls, the initiation of patients with PAI-1 deficiency showed a median lag-time of 57% (p<0.001, IQR 47-66%), and thrombin peak time of 77% (p<0.001, IQR 68-88%); thrombin peak height was low with a median of 67% (p<0.001, IQR 62-80%), with a normal AUC of 106% (IQR 86-124%), a normal fibrin lysis time (median 104%, IQR 80-111%) with high plasmin peak height and plasmin potential (median with IQR 254%, 193-298% and 232%, 182-267%, respectively, both p<0.001).
The NHA results in PLGD and PAI-1 deficient patients as compared to the reference group of healthy controls are displayed in the figure below.
Conclusion:
Patients with either PLGD or PAI-1 deficiency show distinct abnormalities in plasmin and thrombin generation in the NHA. The differences observed in the propagation phase of thrombin generation may be explained by plasmin generation. These results suggest that disorders of fibrinolysis also influence coagulation and a global assay measuring both activities may better correlate with clinical outcome.



Contact:
Joline.Saes@radboudumc.nl