Maria Isabel Bravo1, Aida Raventos1, Alba Perez1, Montserrat Costa1, Todd Willis2
1Discovery Research, Bioscience Industrial Group, Grifols, Barcelona, Spain; 2Discovery Research, Bioscience Industrial Group, Grifols, Raleigh NC, United States
BACKGROUND
Emicizumab (Hemlibra®, Chugai-Roche) is used to bridge aFIX and FX to function as missing aFVIII in hemophilia A (HA) patients. Although limited clinical results are available, fatal thrombotic complications have been reported when using emicizumab combined with activated prothrombin complex (aPCC) but not with recombinant activated factor VII (rFVIIa) or coagulation factor VIII (FVIII)1-2. Therefore, further studies to elucidate the thrombotic effect of combined emicizumab with concomitant haemostatic treatments are still needed.
In this study, we assessed thrombin generation (TG) in vitro when a plasma-derived FVIII/VWF (Alphanate®/Fanhdi®, Grifols) was added to haemophilia A plasma (HAp) with emicizumab. We provided a complementary insight to an abstract presented at ISTH by assessing the percentage of additive effect on TG.
METHODS
- A pool of HA plasma samples without inhibitors (HAp) or individual HA plasma samples with inhibitors (HAp-i) (5-BU), either alone or containing therapeutic doses of emicizumab (50 µg/ml) was combined with aPCC (FEIBA®, Shire) at 0.5 U/ml (approximately equivalent to 25 U/kg), rFVIIa (NovoSeven®, Novo Nordisk) at 0.5 to 7 µg/ml (approximately equivalent to 50 to 700 µg/kg), or pdFVIII/VWF (Alphanate®/Fandhi®, Grifols) at 0.1 to 4.5 IU/ml (approximately equivalent to 5 to 200 IU/kg).
- Samples were analyzed with TG assays using Calibrated Automated Thrombogram (CAT) from Stago:
- Trigger solution (Tissue Factor and Phospholipids), PPP Reagent LOW™
- Fluorogenic substrate and CaCl2, FLUCAkit™
- Fluoroskan Ascent® reader (Thermo Scientific; filters 390‐nm excitation and 460 nm emission)
- Reaction parameter (Peak Height [Thrombin peak]) (Figure 1), calculated using Thrombinoscope™ software
- TG of HAp with emicizumab alone or combined with the different products were compared.

RESULTS
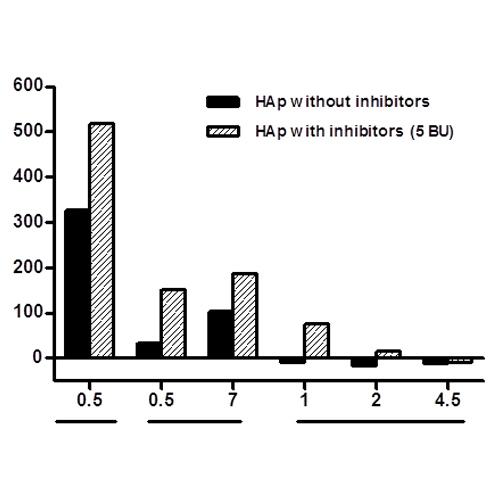
TG for 13 individual healthy plasma samples was evaluated to establish TP normal ranges (47-104 nM). TP for both HAp (Figure 2A) and HAp-i (Figure 2B) in presence of emicizumab showed that addition of aPCC 0.5 U/ml induced a synergic response on TP even at low doses, while rFVIIa moderately increased TP in a dose-related manner but within normal ranges. In contrast, when pdFVIII/VWF was added to HAp or HAp-i, TP was similar to that observed without emicizumab once reached the normal levels.

The absence of additive effects of the concomitant use of pdFVIII/VWF and emicizumab is shown as the percentage of TP increase versus samples without emicizumab (Figure 3).

CONCLUSIONS
After reaching a normal TG range, emicizumab would have a limited ability to promote FX activation in the presence of pdFVIII/VWF, thus reducing the risk of overdosing. Our data support that pdFVIII/VWF has non-additive effects when combined with emicizumab.
REFERENCES:
[1] Oldenburg J et al. N Engl J Med 2017; 377:809-818.
[2] Mahlangu J. N Engl J Med 2018; 379:811-822.
ACKNOWLEDGEMENTS:
The authors thank C. Aparicio, F. Doncel, S. Garcia, C. Gelabert, J. Vidal and E. Vior for their expert technical assistance. Jordi Bozzo and Eugenio Rosado are acknowledged for editorial assistance in the preparation of the poster.