Carmen Escuriola Ettingshausen1 on behalf of the ObsITI Study Group
1HZRM Hämophilie Zentrum Rhein Main GmbH, Mörfelden-Walldorf, Germany
Introduction
- Neutralising antibodies against exogenous factor VIII (FVIII) remain the most significant treatment complication in approximately one-third of previously untreated patients (PUPs)1,2
- Development of a FVIII inhibitor reduces the effectiveness of bleeding control and results in increased morbidity, mortality and socioeconomic burden3-6
- Immune tolerance induction (ITI) is the only clinically proven strategy for eradication of inhibitors and is recommended as the primary treatment option7-10
- The observational ITI (ObsITI) study (NCT02207894) is an ongoing, prospective and retrospective, investigator-initiated, international, observational study systematically documenting patients with haemophilia A and inhibitors undergoing ITI with any FVIII product
- Here we report an analysis on patients prospectively enrolled in the ObsITI study who were treated with the pdFVIII/VWF concentrate octanate®
ITI treatment regimen
- Patients starting ITI according to the Bonn protocol received 50–100 IU/kg daily or every other day (low responders), or 100 IU/kg every 12 h (high responders)
- Bypassing agents (activated recombinant factor VII [rFVIIa] or activated prothrombin complex concentrate [aPCC]) were additionally administered in cases of increased bleeding tendency or acute bleeding during ITI
- Upon achievement of complete success, FVIII dose was slowly tapered down to ≤ 50 IU/kg every other day as prophylaxis
Patients and methods
- Males of any age with haemophilia A and inhibitor titres of ≥ 0.6 Bethesda units (BU)/mL, or < 0.6 BU/mL with reduced FVIII recovery or FVIII half-life are eligible to enter the study
- Patients with congenital or acquired bleeding defects other than haemophilia A, with concomitant immunological diseases, or with a history of hypersensitivity to blood products and/or FVIII concentrates are excluded
- Product choice and treatment regimen for ITI are at the investigator’s discretion; however, the Bonn protocol is the recommended ITI regimen
- The study is coordinated by HZRM Haemophilia Centre Rhine-Main, Germany, and has been approved by the relevant Ethics Committees
- This analysis reports data for patients treated (between December 2005 and January 2019) exclusively with octanate®, a native human, highly purified, VWF-stabilised FVIII concentrate
- Only patients who had completed the study (100 of the 117 patients who started ITI with octanate®) were included in the analysis. Study completion was defined as either achievement of complete success, completion of 36-months’ observational period, or termination of study participation by the investigator
ITI outcomes
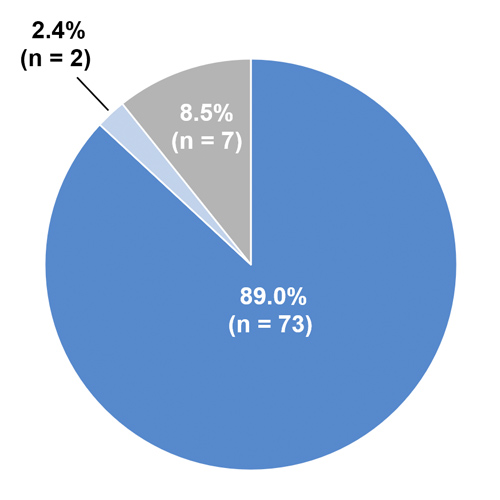
- ITI success was defined as in Table 1, according to achievement of:
- Criterion I: inhibitor titre < 0.6 BU/mL
- Criterion II: FVIII recovery ≥ 66% of the predefined reference value of 1.5% IU/kg ≤ 1 h post injection
- Criterion III: FVIII half-life ≥ 6 h
- Relapse was defined as the reappearance of an inhibitor titre ≥ 0.6 BU/mL at two consecutive time-points within 12 months of achieving complete or partial success and having reached the target prophylactic treatment regimen
- Bleeding episodes (BEs), use of bypassing agents and adverse drug reactions (ADRs) were recorded throughout the study period
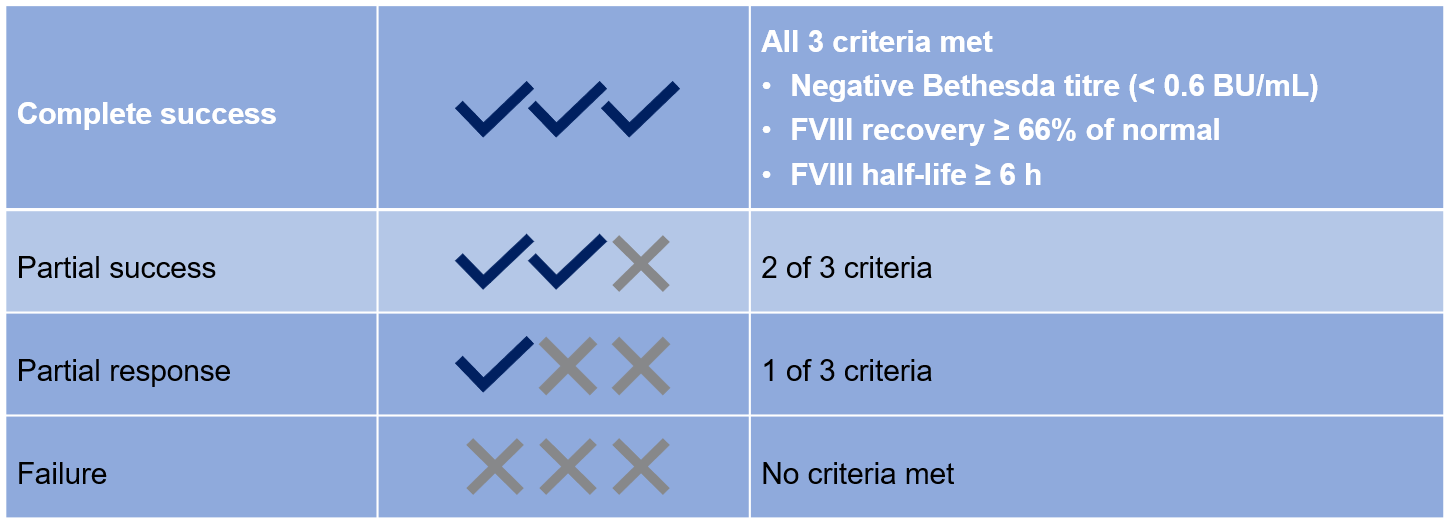
Table 1: ITI success/failure criteria
Results
Patients
- At the time of the analysis, 100 patients at 43 centres in 17 countries had completed ITI with octanate®
- 86 patients (86%) were high responders, 14 (14%) were low responders
- 72 patients (72%) were primary ITI patients, 28 (28%) were rescue ITI patients
- At least one risk factor historically associated with a poor ITI prognosis was present in 91 (91%) patients
- F8 mutation analysis data were available for 82 patients (Figure 1)
- 89% of patients had a high-risk F8 mutation
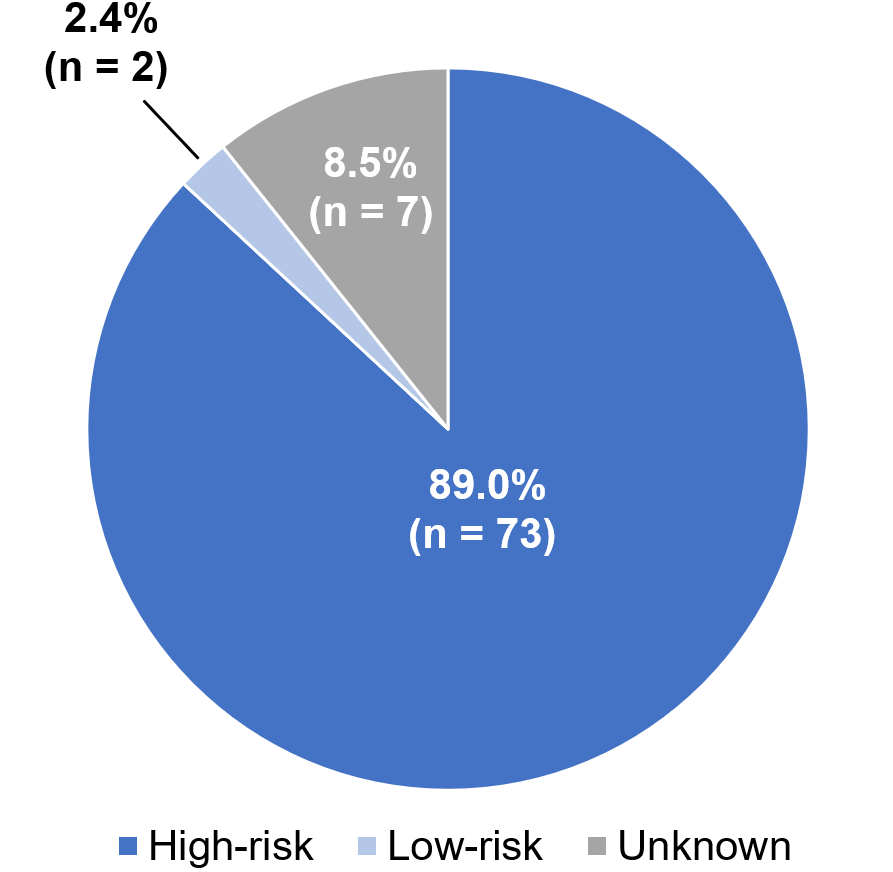
Figure 1: Distribution of high and low-risk F8 gene mutations*
*High-risk mutations: translocations (intron 22 inversion or intron 1 inversion), large deletions, nonsense mutations, splice site mutations, small deletions or small insertions; Low-risk mutations: missense mutations or large duplications
Outcomes
- Inhibitors were eliminated in 71 of 100 (71%) patients
- Complete or partial ITI success was achieved by 70 of 100 (70%) patients (Table 2, Figure 2):
- 56 of 72 (77.8%) primary ITI patients, 14 of 28 (50.0%) rescue ITI patients
- 13 of 14 (92.9%) low responders, 57 of 86 (66.3%) high responders
- The probability of ITI success (Cochran-Mantel-Haenszel test) was higher in primary vs rescue ITI patients (p = 0.01), low responders vs high responders (p = 0.02), and in patients with a baseline inhibitor titre ≤ 10 BU/mL vs > 10 BU/mL (p = 0.001)
- ITI success rates were not significantly different when analysed by time to ITI from inhibitor detection (</≥ 2 years, p = 0.31) or age at start of ITI (</≥ 7 years, p = 0.67)
- No patients relapsed after achieving complete success. One (1.4%) high responder patient with 4 poor prognosis factors had inhibitor reoccurrence within 12 months of achieving partial success
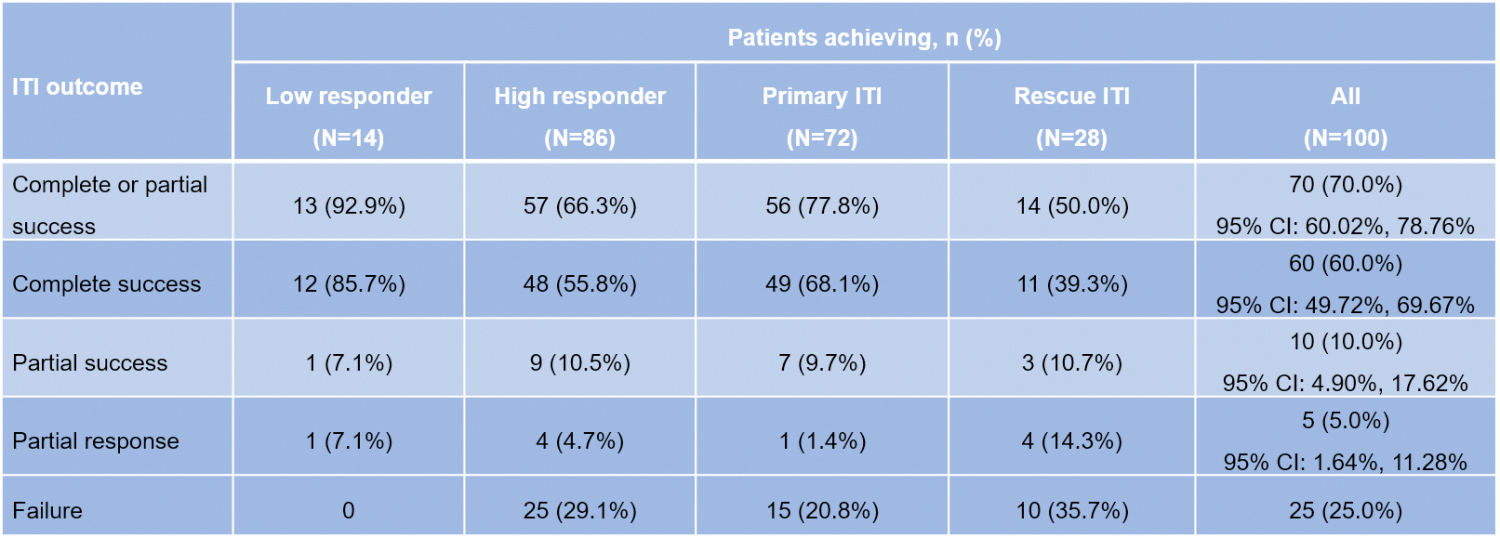
Table 2: ITI success rates with octanate®
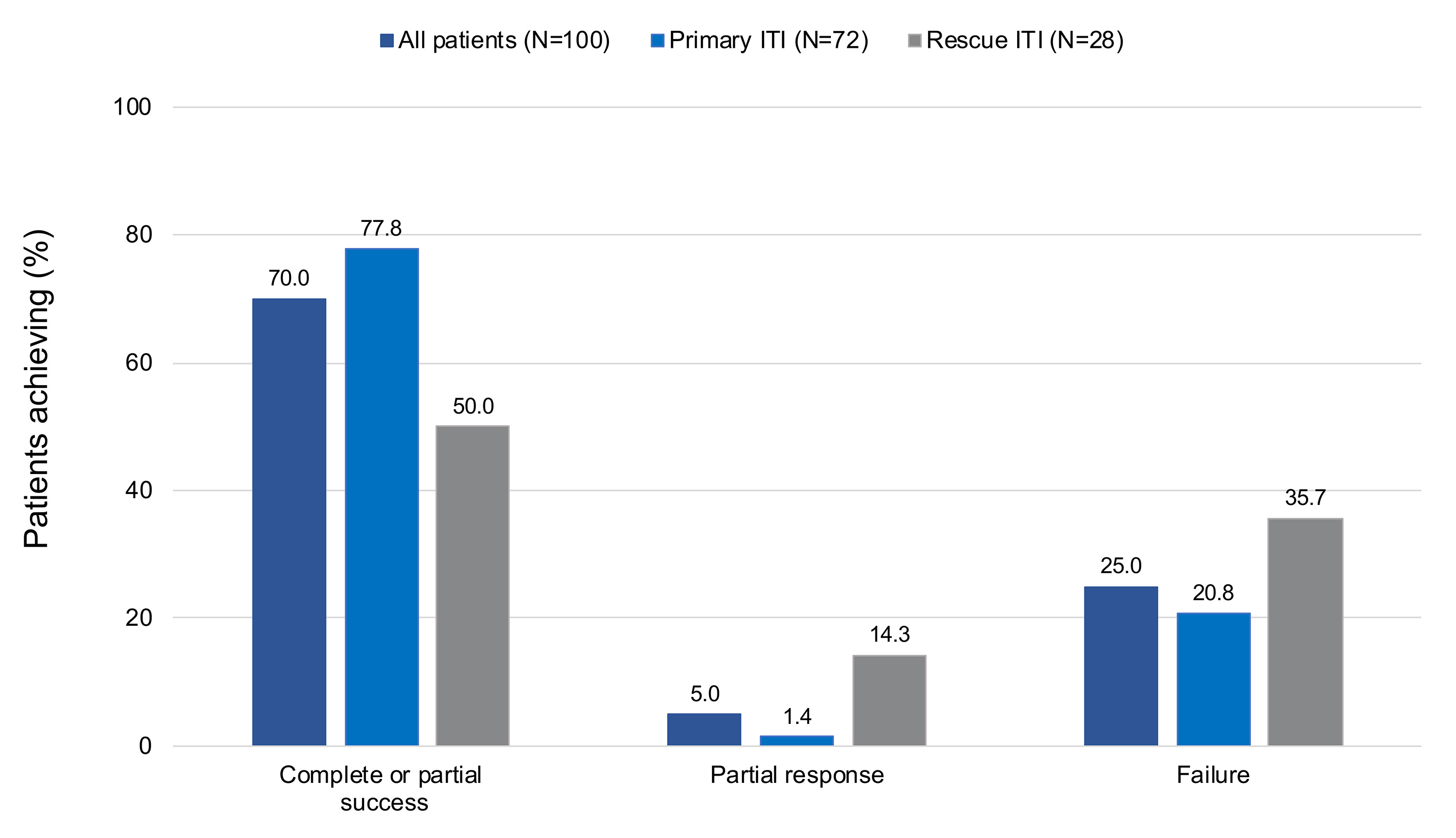
Figure 2: Achievement of ITI outcomes according to primary or rescue ITI
- Inhibitors were eliminated in a median of 4 months, and ITI success was achieved in a median of 12.5 months (Figure 3)
- A statistically significant 93% reduction in mean [SD] monthly bleeding rate was observed following achievement of a negative inhibitor titre (0.45 [0.74] to 0.03 [0.10], p = 0.0002; Figure 4)
- After achieving normalised recovery and half-life, the monthly bleeding rate was not significantly different from the bleeding rate after achievement of a negative inhibitor titre
- The reduction in bleeding was reflected in the decreasing monthly frequency of bypassing agent administration from ITI start to criterion I (mean [SD] 4.11 [9.92]), criterion I to criterion II (1.09 [5.63],
p < 0.0001 vs ITI start to criterion I) and criterion II to criterion III (0.57 [4.21]) - Two serious adverse drug reactions (catheter infections of moderate intensity in the same patient) were considered related to treatment
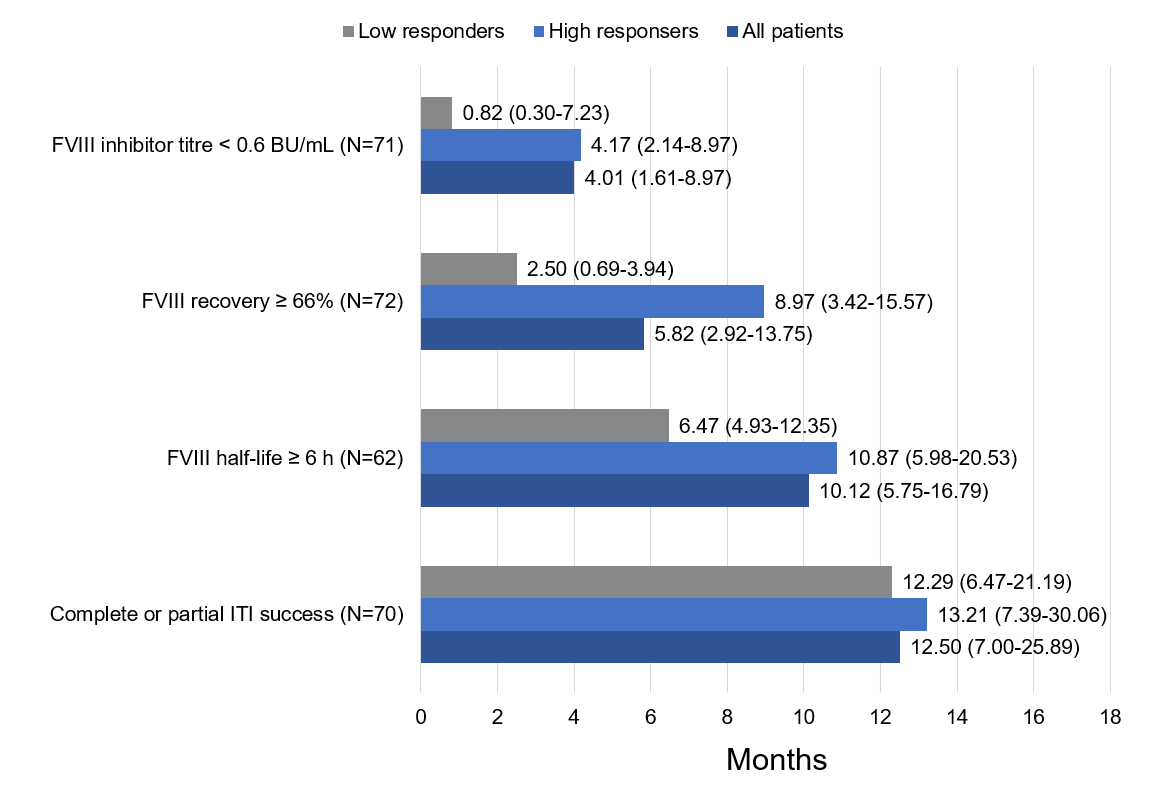
Figure 3: Time-to-achievement of ITI success according to responder type, median (interquartile range)
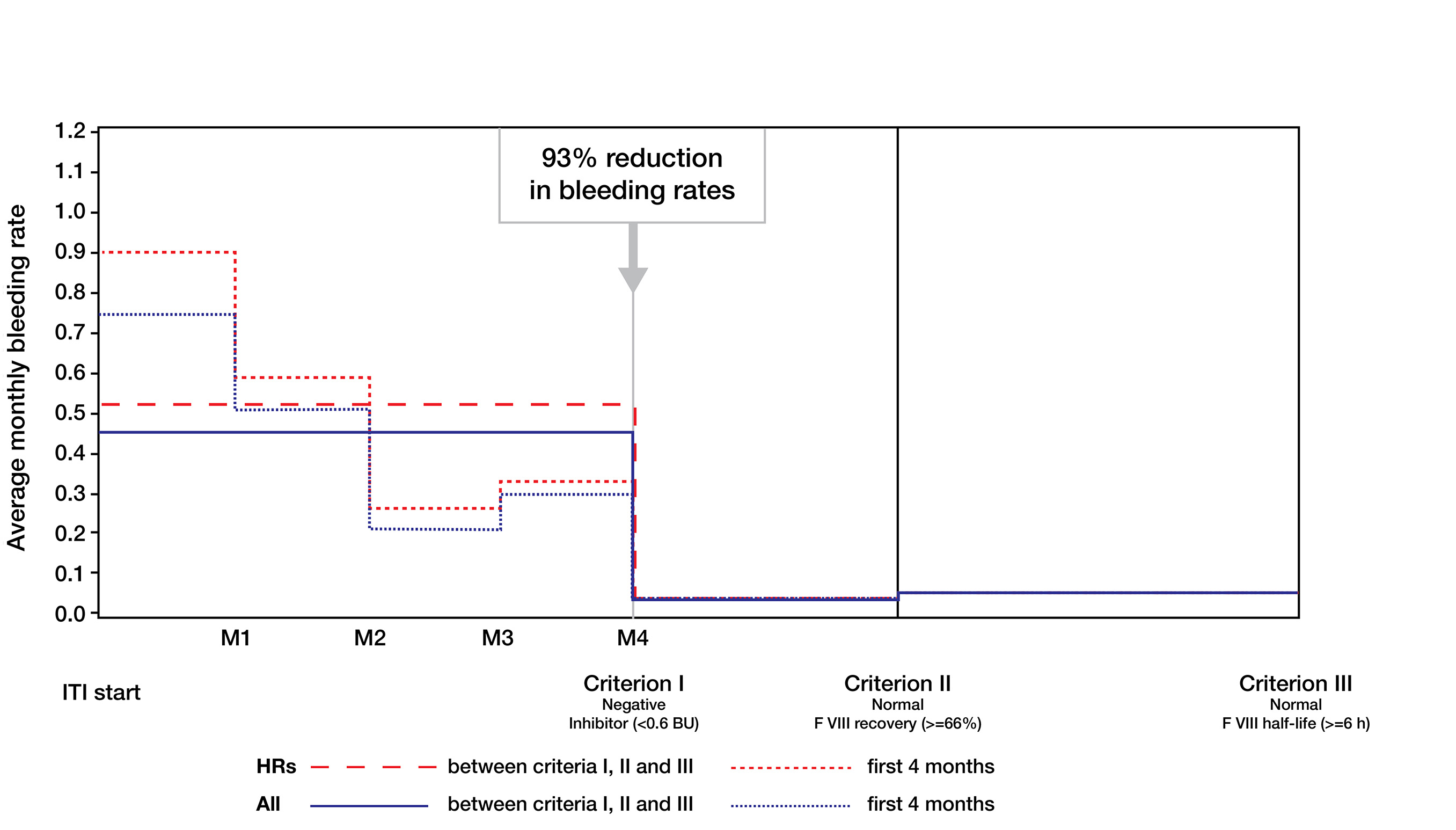
Figure 4: Monthly bleeding rates before ITI and after achievement of criteria I, II and III
Conclusions
- This analysis reports ITI outcomes for 100 prospective patients, most of whom had poor prognosis, undergoing ITI with octanate® and represents the largest prospective cohort with a single product in an observational ITI study
- Inhibitors were eliminated in 71% of patients in a median time of 4.0 months
- ITI success was achieved by 70% of patients in a median time of 12.5 months
- ITI with octanate® led to rapid, sustained eradication of FVIII inhibitors and normalisation of FVIII pharmacokinetics. No relapses after achieving complete ITI success were reported
References
- Gouw SC et al. N Engl J Med 2013; 368:231-9.
- Peyvandi F et al. N Engl J Med 2016; 374:2054-64.
- Darby SC et al. J Thromb Haemost 2004; 2:1047-54.
- Fischer K et al. Haemophilia 2005; 11:43-8.
- Di Minno M et al. Haemophilia 2010; 16:e190-201.
- Walsh CE et al. Thromb Haemost 2016; 116(Suppl. 1): S10-17.
- Giangrande PLF et al. Orphanet J Rare Dis 2018; 13:66.
- Valentino LA et al. Haemophilia 2015; 21:559-67.
- Collins P et al. Haemophilia 2017; 23:654-9.
- Carcao M et al. Haemophilia 2019; 25:676-84.