M. Unterberger, L. Wagner. N. B. Binder
Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH. Vienna. Austria
Introduction
Background
To monitor treatment in haemophilic patients is crucial. It is well known that global assays or FVIII/FIX activity determination do not correlate with bleeding episodes. For patients treated with B domain deleted FVIII preparation (rFVIII) the factor activity determination is difficult. Thrombin generation assay (TGA) measures the entire thrombin generation process and a good correlation in treatment with anti-Inhibitor Coagulant Complex (FEIBA) or rFVIIa has been shown.
Aim
Aim of this study was to show that TGA parameters. measured under standardized conditions can be used with sufficient sensitivity in haemophilic patient samples to monitor replacement therapy with different FVIII preparations.
Materials and Methods
Method: For the experiments we used plasma from normal donors and plasma from Haemophilia A patients. For spiking experiments Haemophilia A patient plasma was spiked at various concentrations with following FVIII preparations:

TGA was determined fully automated on Ceveron t100 with standardized assay kit Ceveron® TGA RB. A TGA calibration curve is made once per substrate lot.
Results
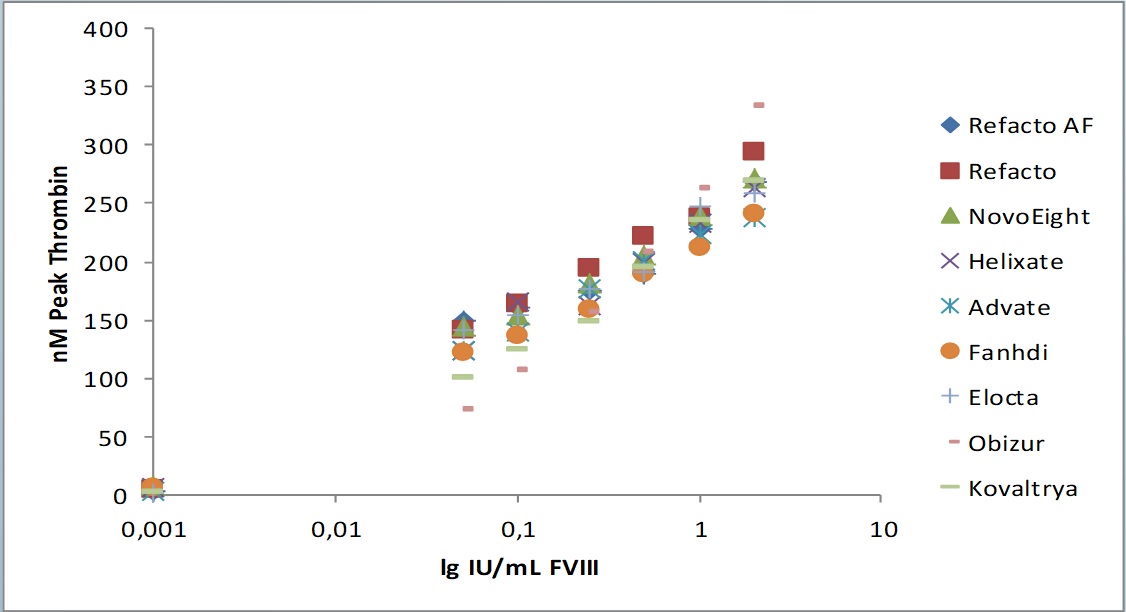
Fig. 1 Haemophilia A sample spiked with increasing concentrations of FVIII preparations showed a continuous increase in Peak Thrombin.
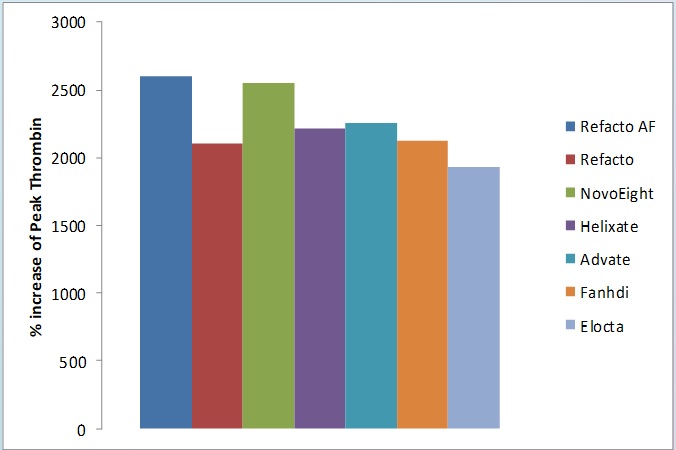
Fig. 2 Haemophilia A sample spiked with increasing concentrations of FVIII preparations showed between 0 and 0.05 IU/mL FVIII a 2000 to 2500% increase of Peak Thrombin value.
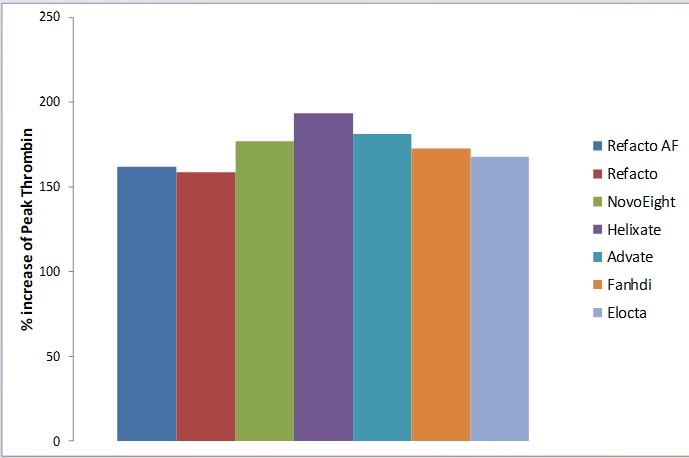
Fig. 3 Between 0.05 IU/mL FVIII and 1.0 IU/mL FVIII of respective FVIII preparation the % increase in Peak Thrombin value was between 150 and 200.
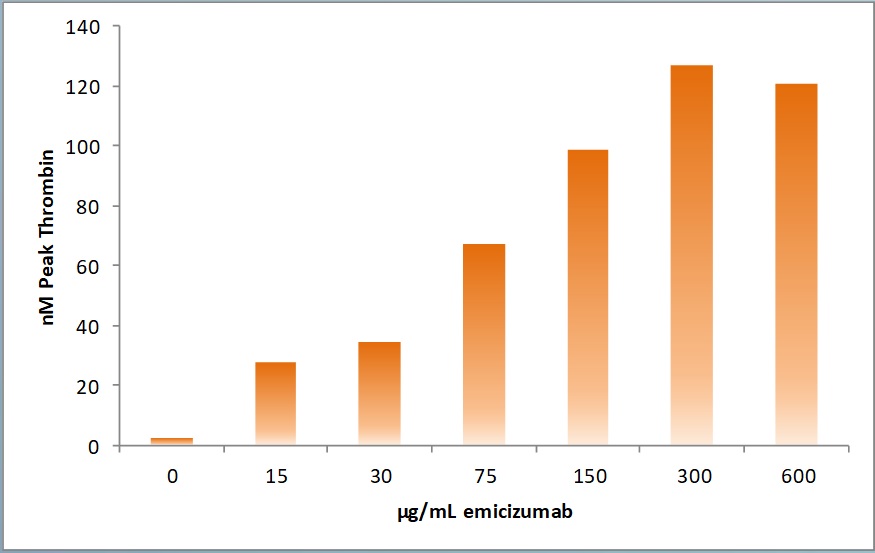
Fig. 4 Haemophilia A sample spiked with increasing concentrations of emicizumab showed a continuous increase in Peak Thrombin.
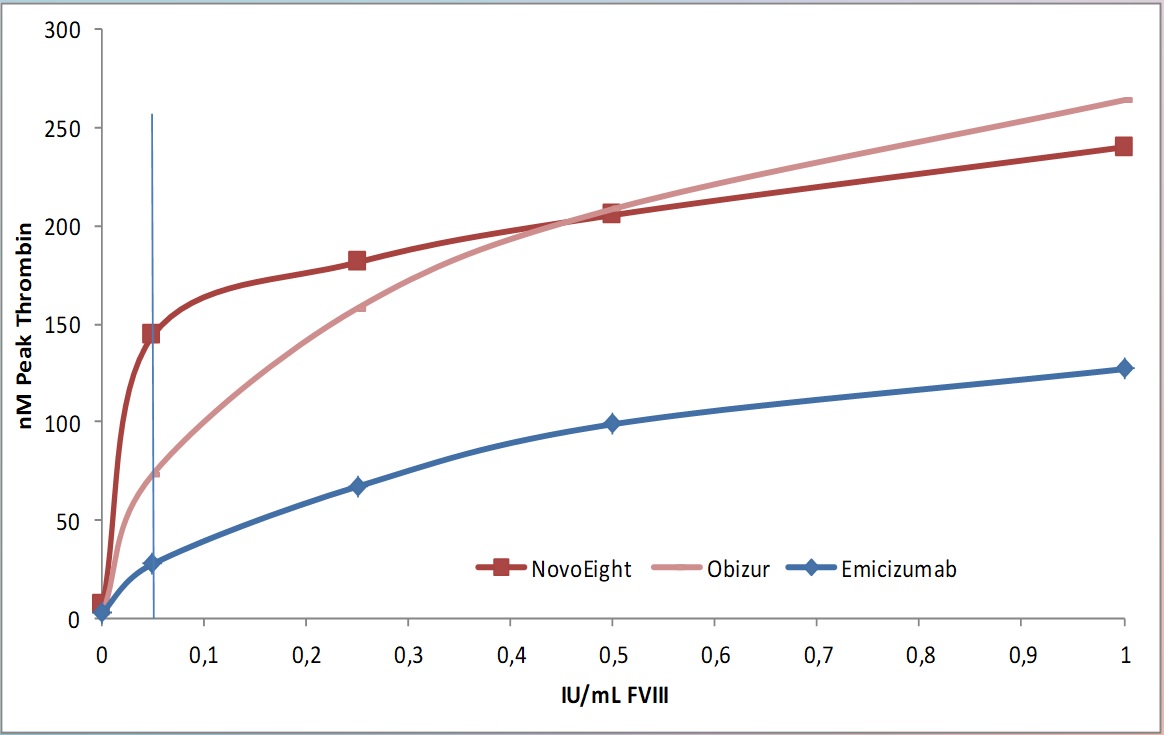
Fig. 5 The initial ~25-fold increase in Peak Thrombin shown in spiking experiments with 0.05 IU/mL B-domain dileted rhFVIII preparation NovoEight, is at ~12-fold in sample spiked with B-domain deleted rpFVIII preparation Obizur and ~ 4-fold in samples spiked with emicizumab.
Conclusions
When TGA is used automated under standardized conditions on coagulation analyzer Ceveron t100 and with the appropriate reagent kits, it is a very sensitive tool to monitor factor replacement therapy and can be used to demonstrate correlation with individual bleeding risk during replacement therapy in clinical studies.
Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH | Brunner Str. 67 |1230 Vienna | Austria |
Tel: +43 1 86 373 0 | Fax: +43 1 86 373 44 | Email: products@technolone.com
