M.T. Pagliari1, L. Baronciani2, G. Cozzi2, P. Colpani2, F. Peyvandi2,3
1Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Università degli Studi di Milano, Department of Pathophysiology and Transplantation and Fondazione Luigi Villa, Milan, Italy; 2Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy; 3Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy
Introduction
von Willebrand factor (VWF) is a large multimeric glycoprotein that is essential for platelet-dependent primary haemostasis1. VWF propeptide (VWFpp) has an important role in the intracellular processing of VWF. Indeed, it takes part in the multimerization process and it regulates the storage of VWF. After secretion into plasma the VWFpp circulates independently from VWF2. VWFpp mutations have been previously associated with types 1, 3 and 2A(IIC) VWD3,4.
We herein describe the characterization of two unrelated Italian patients affected with type 1 VWD (VWD1), carrying the same novel variant localized in the VWFpp.
Aim
The aim of this study was to characterize, at both molecular and functional level, two patients carrying the p.Thr274Pro variant in the VWFpp.
Methods
- Conventional phenotype tests were carried out to evaluate patients’ plasma and platelets as recommended by ISTH-SSC guidelines.
- Molecular analysis of VWF was performed using next-generation sequencing (NGS).
- In silico tools (SIFT, polyphen2.0, SNP&GO, MutationTaster and Pmut) were used to predict the likely damaging effects of this substitution.
- For the in vitro expression studies the pcDNA3.1-VWF-WT and mutant pcDNA3.1-VWF-Thr274Pro expression vectors were transiently transfected, alone and together, into HEK293 cells. The amount of mutant Thr274Pro recombinant (r)VWF and hybrid Thr274Pro/WT-rVWF, which mimics patients’ heterozygous state, were reported as a percentage of the WT-rVWF set as 100% ±SD.
Results
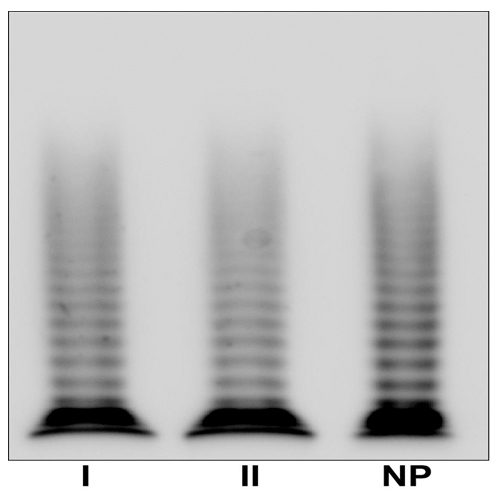
Biochemical analysis of plasma VWF indicated a VWD1 diagnosis (Table 1). Multimeric analysis of patients’ plasma at low resolution showed a normal pattern in both patients (Figure 1; left). The intermediate resolution gel showed a normal triplet structure in the two patients (Figure 1; right).
A novel VWF variant in heterozygous state was identified in patients’ VWFpp (c.820A>C; p.Thr274Pro) using NGS. The presence of the mutation was confirmed by Sanger sequencing.
The potential damaging effect of p.Thr274Pro was predicted by all the in silico tools used and then confirmed with in vitro expression studies. The amount of secreted mutant Thr274Pro-rVWF was reduced to 21±2%, whereas the hybrid Thr274Pro/WT-rVWF was reduced to 36±4% in accordance to patients’ plasma VWF:Ag levels. The amount of rVWF in cell lysates was nearly normal for both Thr274Pro-rVWF and Thr274Pro/WT-rVWF as showed in Table 2.
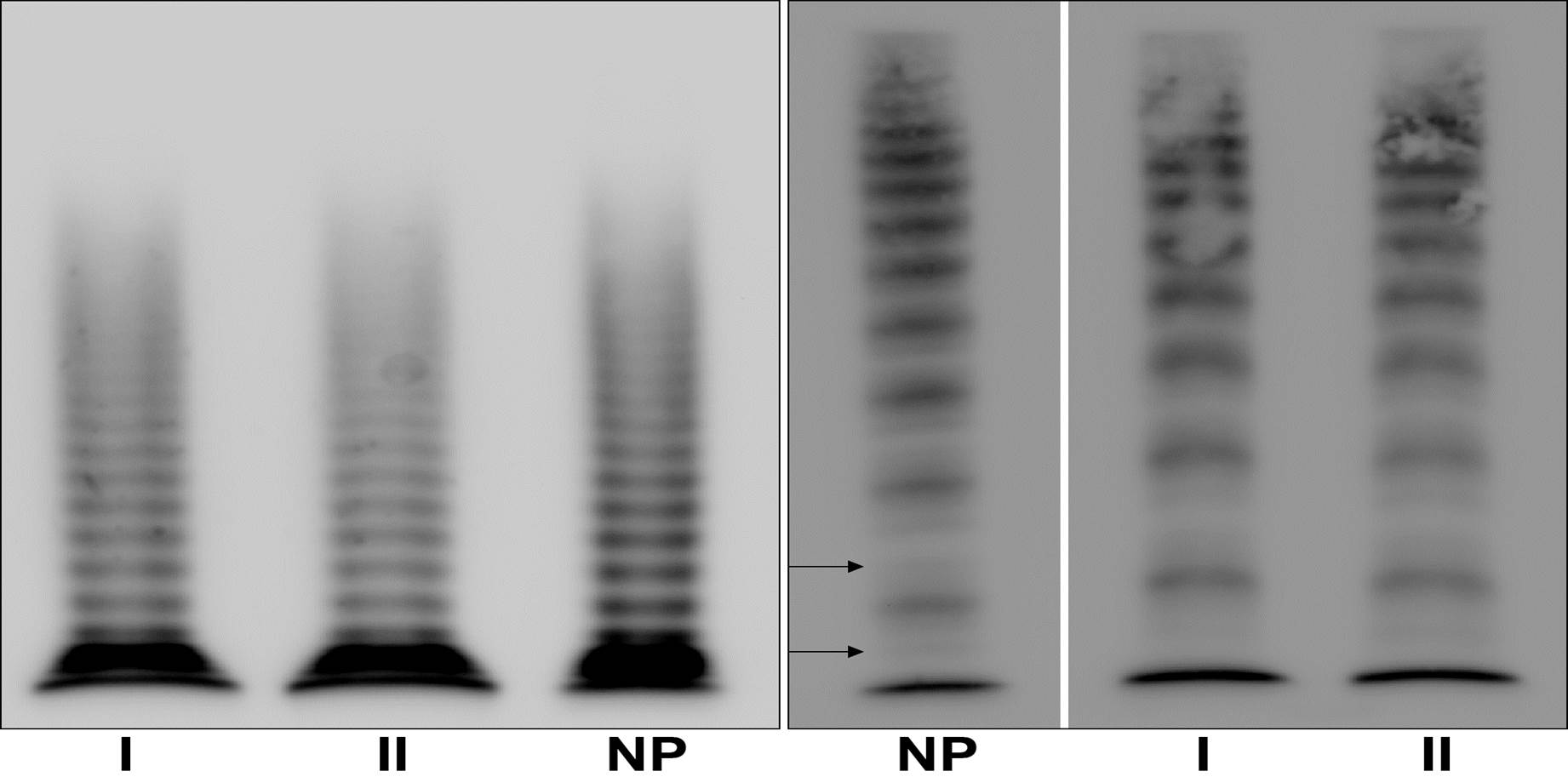
Figure 1. Multimeric analysis of patients’ plasma VWF.
Left: Multimeric structure of plasma von Willebrand factor (VWF) visualized in a non-reducing low-resolution gel (1.2% HGT agarose/0.1% SDS) to highlight the high molecular weight multimers. Right: Multimeric structure of plasma VWF visualized in non-reducing intermediate-resolution gel (1.6% LGT agarose/0.1% SDS), to highlight the triplet structure. NP, normal plasma. NP-VWF showed the typical triplet structure and the satellite bands are indicated by the arrows. Lanes from the same gel are delimited by a black line.
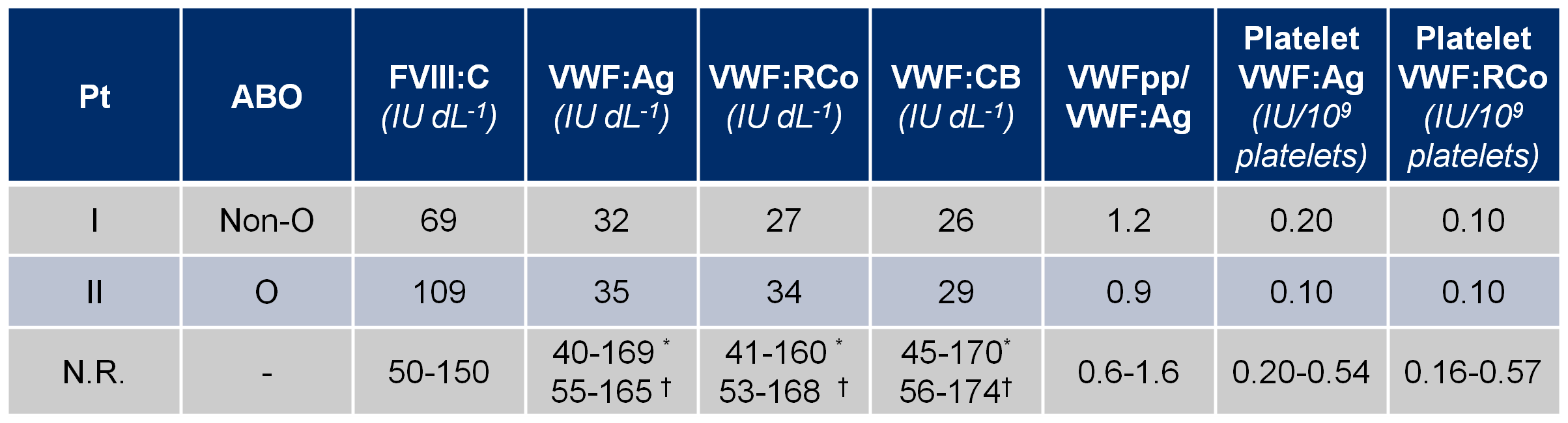
Table 1. Biochemical data of the two patients.
Pt, patient; ABO, blood group system; FVIII:C, factor VIII coagulant activity; VWF:Ag, von Willebrand factor antigen; VWF:RCo, von Willebrand factor ristocetin cofactor activity; VWF:CB, von Willebrand factor collagen-binding activity; VWFpp, von Willebrand factor propeptide; N.R., normal range. * range values of normal individuals with blood group O; † range values of normal individuals with blood group non-O. Values are shown as a mean of three measurements in three different samples. Patients’ platelets were isolated once.
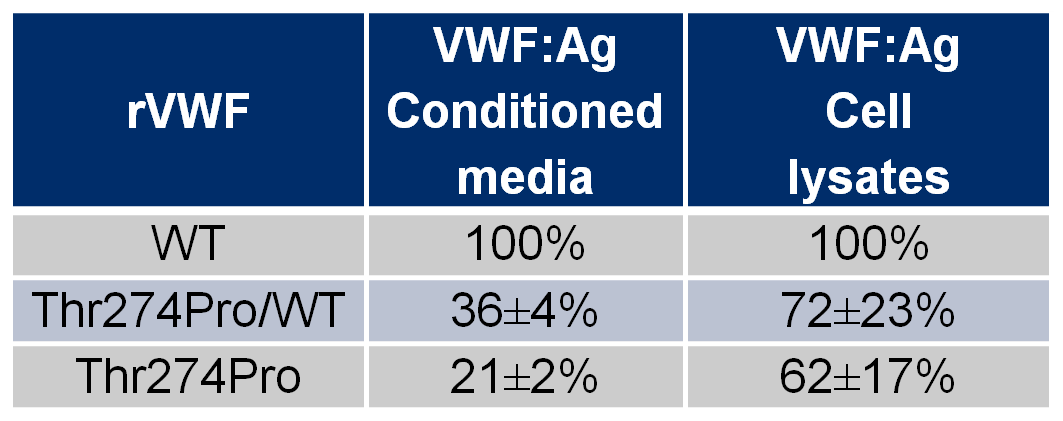
Table 2. In vitro expression study of Wild-type, hybrid and mutant recombinant von Willebrand factor.
rVWF, recombinant von Willebrand factor; WT, Wild-type. VWF:Ag, von Willebrand factor antigen. The amount of hybrid (WT/mutant) and mutant rVWF were expressed as a percentage of the WT referred as 100% (±SD).
Conclusions
We identified a novel VWF mutation localized in the VWFpp region in two unrelated Italian patients, using NGS. In silico tools and the in vitro expression studies showed a pathogenic dominant effect of p.Thr274Pro, in accordance with the VWD1 diagnosis of the patients.
References
1 Sadler JE. Biochemistry and genetics of von Willebrand factor. Annual Rev Biochem 1998;67:395–424.
2 Wagner DD, Saffaripour S, Bonfanti R, et al. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991;64(2):403-413.
3 Rosenberg JB, Haberichter SL, Jozwiak MA, et al. The role of the D1 domain of the von Willebrand factor propeptide in multimerization of VWF. Blood. 2002;100(5):1699-1706.
4 Allen S, Abuzenadah AM, Hinks J, et al. A novel von Willebrand disease-causing mutation (Arg273Trp) in the von Willebrand factor propeptide that results in defective multimerization and secretion. Blood. 2000;96(2):560-568.