F. Bridey1, J. Goudemand2, A. Borel-Derlon3, A. Federici4, C. Henriet1 and on behalf of the WILFACTIN Study Investigators
1Laboratoire français du Fractionnement et des Biotechnologies, Clinical Development, Les Ulis, France; 2Haematology Unit, University of Lille, Lille, France; 3Haemophilia Treatment Centre, University Hospital of Caen, Caen, France; 4Department of Oncology and Onco-Haematology, University of Milan, Milan, Italy
INTRODUCTION
WILFACTIN®/WILLFACT® is a plasma-derived von Willebrand factor (pdVWF) concentrate almost devoid of factor VIII (FVIII)*. The concept of a FVIII-poor VWF concentrate is based on the principle that what is missing in VWD patients is VWF only and not FVIII. It was designed to minimize the risk for thrombotic complications if repeated dosing is needed.
AIMS
This analysis aims to present the pooled efficacy and safety results from six prospective studies performed in patients with VWD in which WILFACTIN® was used in the prevention and control of bleeding during surgery.
RESULTS
A total of 153 unique patients with inherited VWD underwent 240 surgical procedures including delivery: 130 procedures performed in 83 patients with VWD Type 2 (54.2%), 65 in 35 patients with VWD Type 3 (27.1%), 41 in 31 patients with VWD Type 1 (17.1%), and 4 in 4 patients with unknown VWD Type (1.7%).
- 75.1% of patients had basal VWF:RCo ≤15 IU/dL and 71.2% of patients FVIII:C <40 IU/dL
- 11.7% of patients were <12 years of age and 13.3% of patients ≥65 years at time of surgery
A wide variety of procedures were performed, however these mainly included dental (68), orthopaedic (41), digestive (35), general (30), gynaecological (22), and 24 childbirths. Seventeen procedures were performed in obese patients with body mass index ≥30 kg/m².
Of the 240 procedures, 147 were to be assessed for haemostatic efficacy. Assessments were available for 146 procedures.
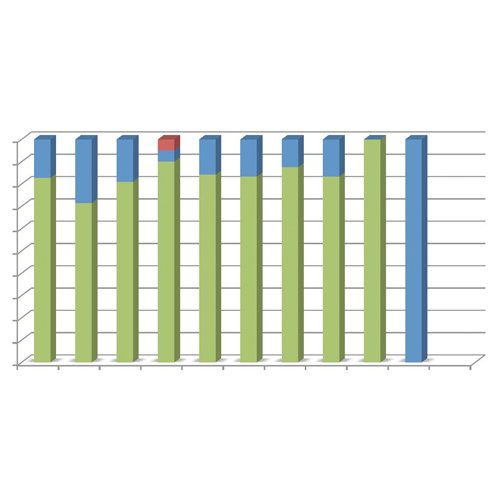
⇒ Efficacy of WILFACTIN® was rated as excellent or good in 99.3% of surgeries (81.5% of surgeries were rated as excellent). One procedure was rated as moderate (caesarean with immediate post-partum haemorrhage resumed in operating suite) (figure 2).
The characteristics of patients, procedures and product dosing are shown in figure 3.
METHODS
Surgical data from five phase 2-3 clinical studies and one post-marketing study carried out between 1999 and 2014 were pooled. Patients received VWF to prevent bleeding and achieve haemostasis during surgery including childbirth. Invasive diagnostic procedures without biopsy were excluded from this analysis. The haemostatic efficacy, on a 4-point scale, was evaluated using the investigator’s assessment of overall haemostasis before hospital discharge. Treatment schedules are shown in figure 1.
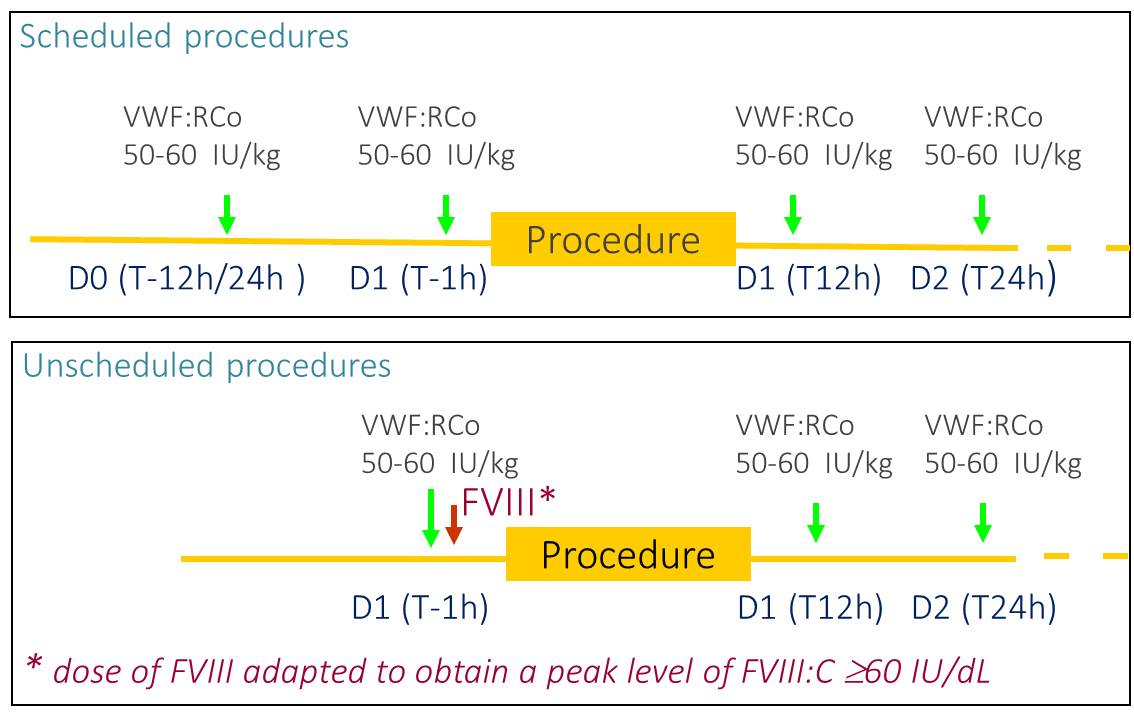
Figure 1: Per protocol recommendations for dosing
Pre-operative Regimen may differ depending on whether or not the surgery was scheduled
Post-operative Regimen used WILFACTIN® as monotherapy: doses to be adapted according to the residual levels of VWF:RCo ≥50-60% and/or FVIII ≥40-50% until complete healing
Safety was assessed by inhibitors to VWF and adverse events (AEs).
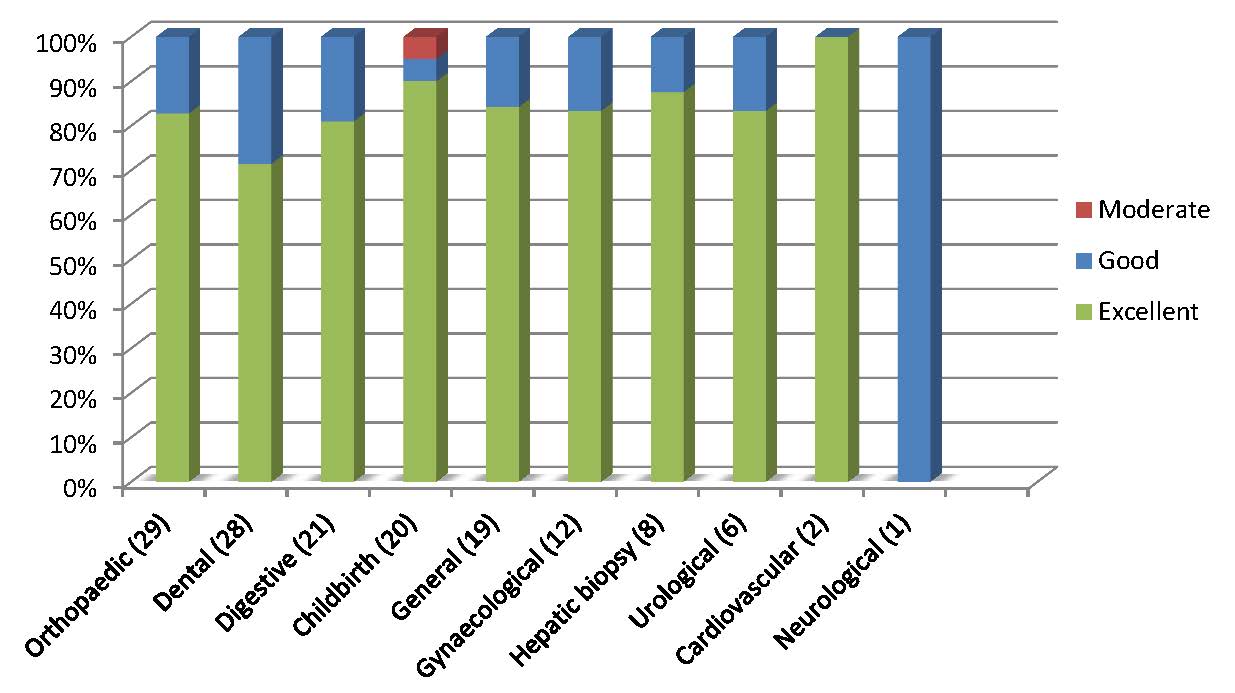
Figure 2: Overall efficacy assessment by surgery type.
Correction of FVIII:C levels was obtained by FVIII in combination with the VWF preoperative dose in 31/147 (21.1%) surgeries. In the 112 remaining procedures for which detailed information was available, WILFACTIN® was administered 12/24h before surgery allowing endogenous FVIII:C correction (68), or FVIII:C levels were considered high enough to ensure haemostasis (44).
SAFETY
There were no drug-related serious AEs following administration of study medication. No severe allergic reactions and no inhibitors to VWF were observed.
No thrombotic events were reported including in the elderly (18 patients between 65 to 85 years, 32 surgical procedures), the obese (17 interventions) or following 41 orthopaedic procedures.
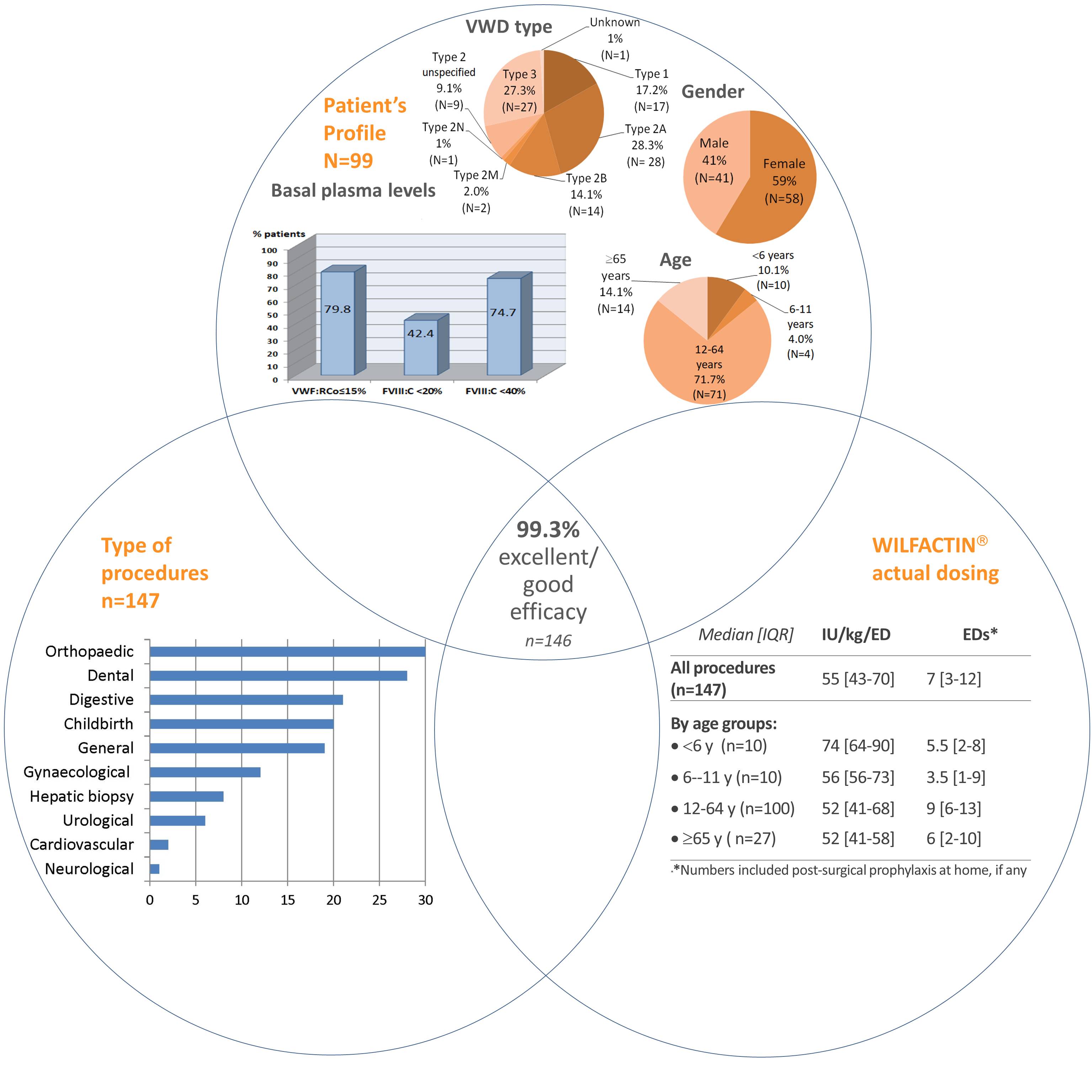
Figure 3: Demographic characteristics, type of procedures and dosing associated with efficacy rating
CONCLUSION
In this clinical overview, the safety and efficacy of a pdVWF product almost devoid of FVIII (WILFACTIN®) was judged over an extended time frame and in a large cohort of patients in the perioperative setting. The results confirm the excellent efficacy of WILFACTIN® in all types of VWD across a range of surgical procedures, including childbirths. These results also provide clinical evidence for the excellent tolerability and long-term safety when used in patients with increased thrombotic risks.
Acknowledgements
The authors wish to thank all the physicians from around the world who contributed clinical or laboratory data on their patients
Aronis-Vournas S, Badowska W, Berger C, Berntorp E, Bertrand MA, Beurrier P, Borel-derlon A, Borg JY, Boyer-Neumann C, Brunot-Ojeda A, Chambost H, Claeyssens S, Durin A, Faradji A, Federici A, Fressinaud E, Gadisseur A, Gay V, Goudemand J, Gouider E, Gruel Y, Guerin V, Guerois C, Guillet B, Hermans C, Klukovska A, Lambert T, Laubriat M, Lavigne G, Lee C, Lorenzini JL, Maes P, Mannucci PM, Marques-Verdier A, Matysiak M, Meddeb B, Moreau P, Negrier C, Nguyen P, Pan-Petesch B, Peerlinck K, Peynet J, Polack B, Rothschild C, Sanderson F, Scharrer I, Schved JF, Sie P, Stieltjes N, Torchet MF, Van Geet C, Vicariot M, Voyer AL, Windyga J.
WILFACTIN®/WILLFACT®
- a single pdVWF concentrate.
- almost devoid of FVIII (≤0.1 IU FVIII / 1 IU VWF:RCo)
- 3 dedicated virus inactivation/removal steps (SD, dry heat, 35nm filtration)
- designed specifically for VWF-deficient patients
The product was first approved in France in 2003 and subsequently (beginning in 2009) 30 countries worldwide, including Italy.