Luciano Baronciani1, Ulrich Budde2, Francesca Stufano1, Flora Peyvandi1,3, Anne Goodeve4, Reinhard Schneppenheim5, Zahra Badiee6, Mohammad-Reza Baghaipour7, Javier Battle8, Erik Berntorp9, Imre Bodó10, Giancarlo Castaman11, Jeroen C.J. Eikenboom12, Peyman Eshghi13, Cosimo Ettorre14, Jenny Goudemand15, Wolf Hassenpflug16, Charles Richard Morris Hay17, Hamid Hoorfar18, Mehran Karimi19, Bijan Keikhaei20, Riitta Lassila21, Frank W.G. Leebeek22, Maria Fernanda Lopez Fernandez8, Pier Mannuccio Mannucci1, Maria Gabriella Mazzucconi23, Massimo Morfini11, Johannes Oldenburg24, Rafael Parra Lòpez25, Ian Peake26, Andreas Tiede27, Gholamreza Toogeh28, Alberto Tosetto29, Marc Trossaert30, Sayed Omid Reza Zekavat31, Eva M.K. Zetterberg32 and Augusto B Federici33
1Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 2MEDILYS Labor Gesellschaft, Hamburg, DEU; 3Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy; 4Sheffield Diagnostic Genetics Service, Sheffield Children’s NHS Foundation Trust, Sheffield, United Kingdom; 5Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; 6Hemophilia-Thalassaemia Center of Mashhad (Sarvar Clinic), Mashhad University of Medical Science, Mashhad, Iran (Islamic Republic of); 7Iranian Hemophilia Comprehensive Treatment Centre, Iranian Hemophilia Society, Tehran, Iran (Islamic Republic of); 8Complejo Hospitalario Universitario de A Coruña – Servicio de Hematología y Hemoterapia, A Coruña, Spain; 9Centre for Thrombosis and Haemostasis, Skane University Hospital, Malmo, SWE; 10Hematology and Stem Cell Transplantation, St. Istvan & St. Laszlo Hospital of Budapest, Budapest, Hungary; 11Hemophilia and Thrombosis Center, Azienda Ospedaliera Universitaria Careggi, Firenze, Italy; 12Department of Thrombosis and Hemostasis, Leiden Univ. Medical Center, Leiden, NLD; 13Mofid Comprehensive Care Centre for Children with Hemophilia, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Islamic Republic of); 14Hemostasis and Thrombosis Center, Azienda Ospedaliera Policlinico Consorziale, Bari, Italy; 15Institut d’Hématologie – Hôpital Cardiologique – University of Lille – Haematology Department, Lille, France; 16Klinik und Poliklinik für Pädiatrische Hämatologie und Onkologie, Hamburg, Germany; 17Manchester Royal Infirmary, Manchester Royal Eye Hospital, Manchester, United Kingdom; 18Hemophilia Center, Esfahan University of Medical Science, Esfahan, Iran (Islamic Republic of); 19Hematology Research Center, Nemazee Hospital, Shiraz University of Medical Science, Shiraz, Iran (Islamic Republic of); 20Research Center for Thalassemia & Hemoglobinopathy – Division of Hematology & Oncology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Islamic Republic of); 21Department Internal Medicine, Coagulation Disorders, at Haematology and Laboratory Services, Helsinki University Central Hospital, Helsinki, Finland; 22Department of Hematology, Erasmus Medical Center, Rotterdam, NLD; 23Hemophilia and Thrombosis Center, University of Rome, Roma, Italy; 24Institute of Experimental Haematology & Transfusion Medicine, University Clinic Bonn, Bonn, Germany; 25Unidad de Hemofilia, Hospital Universitari General Vall d’Hebron, Barcelona, Spain; 26Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, GBR; 27Department of Haematology, Haemostasis, Oncology and Stem Cell Transplantation – Hannover Medical School – Haemophilia Care Centre, Hannover, Germany, 28Thrombosis Hemostasis Research Center – Vali-Asr Hospital – Emam Khmeini Complex Hospital, Tehran University of Medical Science, Tehran, Iran (Islamic Republic of); 29Hemophilia and Thrombosis Center, Ospedale San Bortolo of Vicenza, Vicenza, Italy; 30Centre Régional de Traitement de l’Hémophilie – Laboratoire d’Hématologie, Nantes, France; 31Nemazee Hospital Hemophilia Center, Shiraz University of Medical Sciences, Shiraz, Iran (Islamic Republic of); 32Centre for Thrombosis and Haemostasis, University Hospital Skane, Malmö, SWE; 33Hematology and Transfusion Medicine, L. Sacco University Hospital & School of Medicine, Milan, Italy.
Background
Type 3 VWD (VWD3) is inherited with an autosomal recessive pattern and is the least common form of VWD in developed countries (affecting 1 in 1 million people). VWD3 is characterized by virtually undetectable levels (<5 IU/dL) of VWF in plasma and very low plasma levels of FVIII. VWD3 patients therapeutic approach consists of plasma derived or recombinant VWF infusions. Following these infusions, approximately 5 to 10% of patients with VWD3 develop alloantibodies against VWF. This event not only complicates patients’ therapy, but also exposes patients to the risk of a life-threatening anaphylactic shock.
Aims
Our aim was to evaluate the presence of inhibitors against VWF in a cohort of European and Iranian patients with previously diagnosed VWD3 enrolled in the 3WINTERS-IPS.
Materials and Methods
Among the 260 VWD3 patients enrolled in the project 205 had plasma VWF antigen (VWF:Ag) <5% and in 151 of these the VWF:Ag was undetectable (<1%) (Figure 1).
We used an enzyme-linked immunosorbent assay (ELISA) approach to detect the presence of anti-VWF antibodies (IgG, IgA and IgM) in patients plasma. Positive samples were re-evaluated using a confirmation test.
The Bethesda method was used to test all patients for the presence of inhibitors using the VWF collagen binding (VWF:CB) assay. The assay was done by performing VWF:CB activity in patient-normal pool plasma mixtures after 1 hour incubation at 37°C. The titre of anti-VWF inhibitor was calculated by the current dilution of VWD plasma inhibiting 50% of normal plasma pool diluted 1:2 compared to control mixture.
Patients with VWF:CB inhibitors were also evaluated by Bethesda method using an automated gain-of-function mutant glycoprotein Ib binding (VWF:GPIbM) and the VWF:Ag assays.
Results
Neutralizing antibodies for VWF:CB were identified in 13 out of 205 (6%) patients and most of them have the VWF:Ag <1% (Table 1). Among them, 10 patients (77%) were positive to the Bethesda VWF:GPIbM and 6 (46%) for Bethesda VWF:Ag assays. All patients with inhibitors present mutations that results in null alleles with one exception, a case who appears to have only a homozygous missense mutation (Table 1).
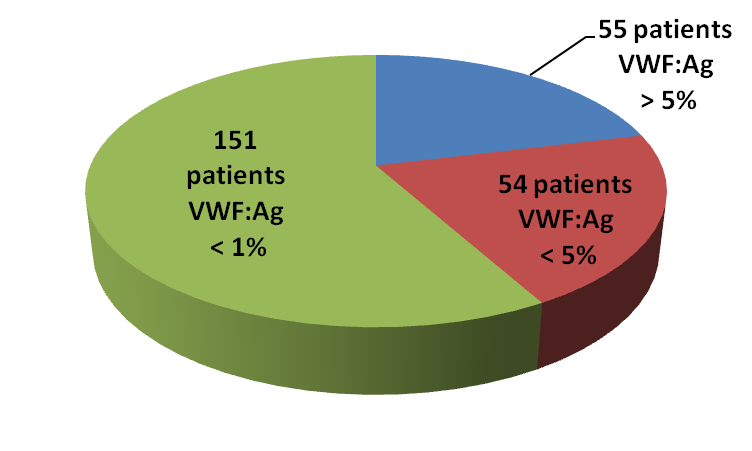
Figure 1.
VWF:Ag levels in a cohort of European and Iranian patients with previously diagnosed VWD type 3 enrolled in the 3WINTERS-IPS project.
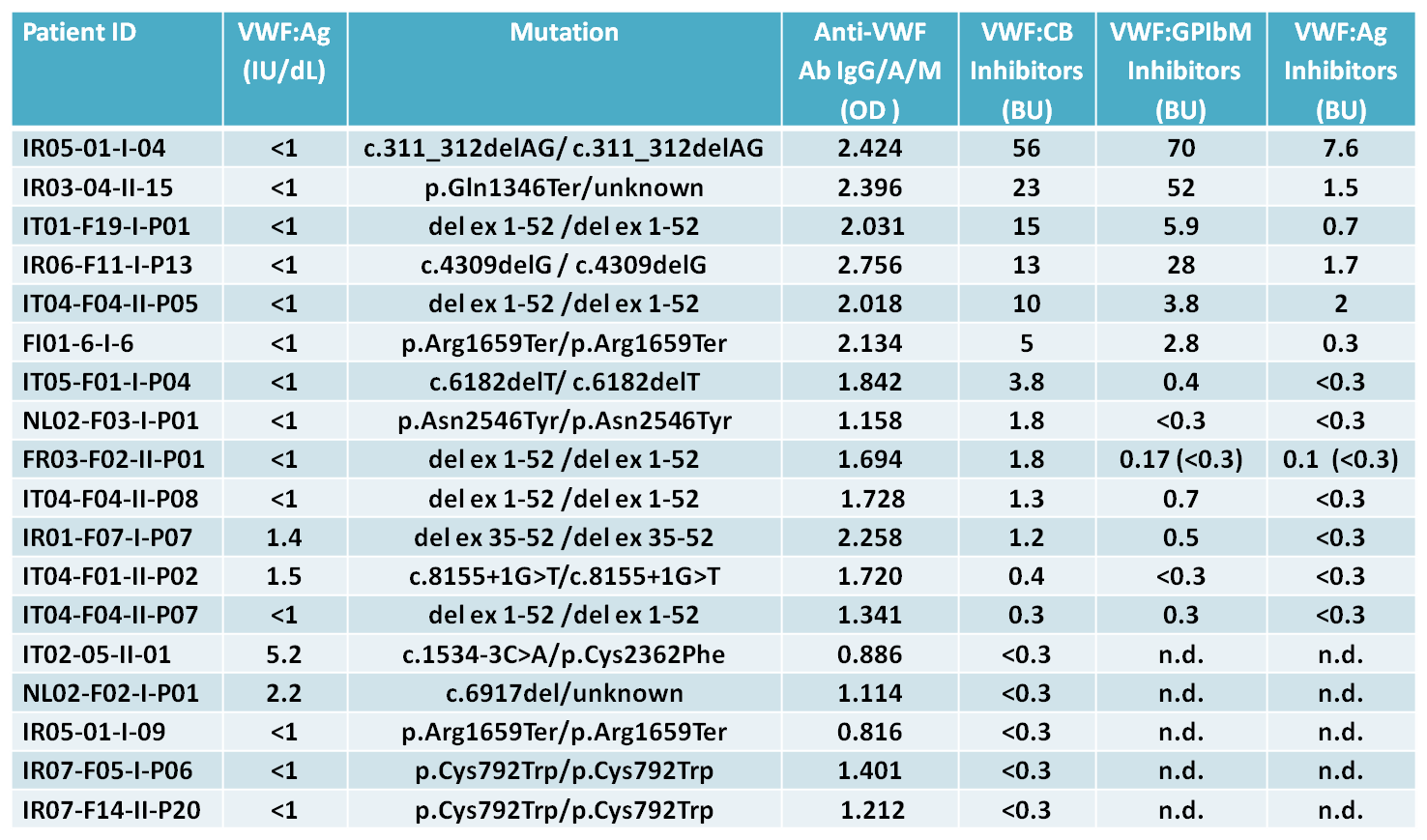
Table 1.
The inhibitors were considered to be present for values ≥ 0.3 Bethesda units (BU); n.d. not determined.
Conclusions
The development of inhibitors in VWD3 patients is confirmed to be a rare event, only 6% of our patients were found to be positive. The use of an ELISA assay to detect anti-VWF antibodies might be useful, but mixing tests using patient-normal pool plasma are important to identify the presence of VWF neutralizing antibodies. High-titer anti-VWF antibodies have been reported to be able to precipitate VWF in normal plasma, this would explain the positive results obtained in six of our patients using the VWF:Ag Bethesda assay. Development of inhibitors is mainly restricted to patients with null alleles and nearly half of them carry homozygous large VWF gene deletions.