Colpani Paola1, Baronciani Luciano1, Stufano Francesca1, Boscarino Marco1, Bucciarelli Paolo1, Pagliari Maria Teresa2, Cozzi Giovanna1, Peyvandi Flora1,3
1Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy; 2Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Università degli Studi di Milano, Department of Pathophysiology and Transplantation and Fondazione Luigi Villa, Milan, Italy; 3Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy
INTRODUCTION
In the last few years alternative assays to evaluate platelet-dependent VWF activity have emerged as alternative to VWF:RCo assay. This test suffers of high inter-assay variation [1] and has some limitations as the demonstrated artefact with specific polymorphisms that prevent ristocetin binding to VWF A1 domain [2]. In a previous study we found a good agreement of results between our VWF:GPIbM ELISA and a VWF:RCo assay, with the exception of type 2B patients, where the values obtained with the VWF:GPIbM ELISA were higher than those obtained using the VWF:RCo assay [3].
AIMS
To compare the results of four different platelet-dependent VWF activity assays in an heterogeneous group of type 2 von Willebrand disease (VWD) patients well characterized (phenotype and genotype).
To evaluate the automated VWF:GPIbM assay in a group of type 2B VWD patients.
PATIENTS
76 VWD patients previously well characterized and classified as (type 2A [n=26], type 2B [n=29], type 2M [n=9] and type 2A/2M [n=12]). Among the 29 type 2B patients 8 were type 2B New York.
METHODS
All samples were tested using four different methods: two VWF:GPIbM assays (one automated: INNOVANCE® VWF Ac, Siemens Healthcare Diagnostics, Marburg, Germany and one home-made ELISA [3]) and two VWF:RCo assays (one automated that use lyophilized platelets [BC Von Willebrand Reagent, Siemens, Marberg, Germany] and one performed with a two-channel aggregometer [Chrono-log Corporation] that use formalin-fixed platelets).
The correlation between all the four assays was calculated using the Pearson’s method, whereas, Bland and Altman plots were used to graphically assess the agreement between the methods by plotting the differences between the values of the two compared techniques against the averages of the values.
RESULTS
The correlation between the four different assays are reported in Table 1. We obtained a good correlation with Pearson’s r > 0.90 between three VWF assays: the two VWF:RCo and the VWF:GPIbM automated assays. The correlation between VWF:GPIbM ELISA and the other assays showed a Pearson’s r ≤ 0.90.
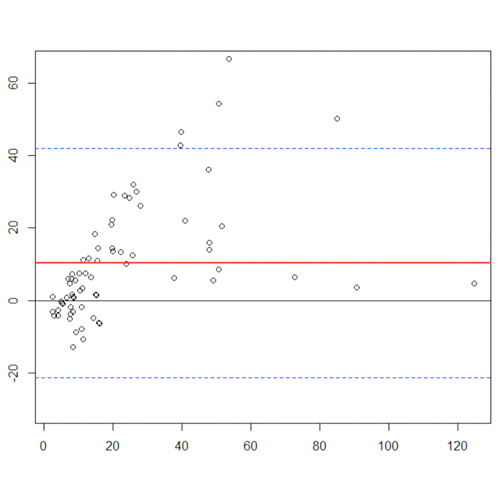
As assessed in Figure 1 by the method of Bland-Altman plots the two VWF:RCo assays exhibited a good agreement (Figure 1D). Similar results, although with few outliers, were obtained when we compared VWF:GPIbM automated versus VWF:RCo assays (Figure 1E-F). At last when we plotted the differences between VWF:GPIbM ELISA against the VWF:GPIbM automated and the VWF:RCo assays (Figure 1A-B-C) we obtained a lower agreement. All the outliers shown in the Bland-Altman plots were type 2B VWD patients with loss of high molecular weight multimers (HMWM). In most of type 2B VWD patients the VWF:GPIbM automated assay showed values remarkably lower that those obtained using the VWF:GPIbM ELISA assay. Furthermore, in some type 2B patients, the VWF:GPIbM automated assay showed lower results in comparison with the two VWF:RCo assays.
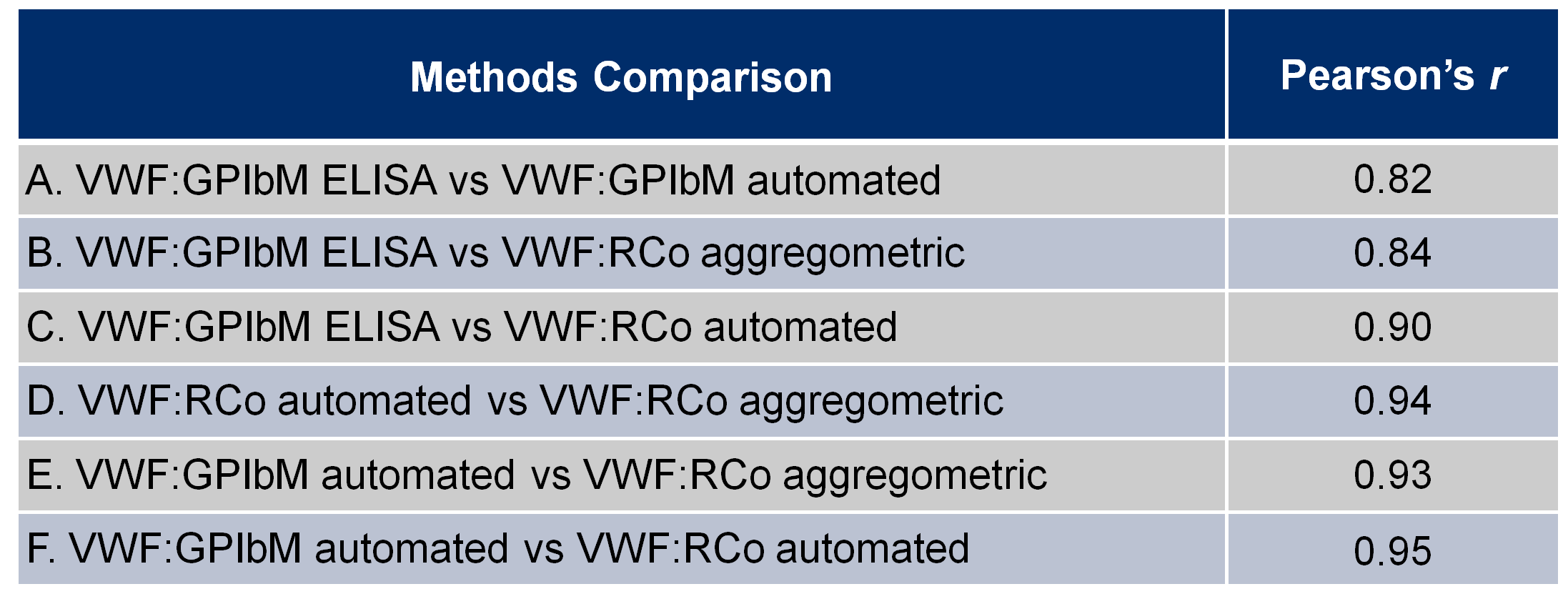
Table 1. Comparison between the alternative assays to evaluate platelet-dependent VWF activity
VWF, von willebrand factor; VWF:GPIbM, all assays that are based on the spontaneuos binding of a VWF to a gain-of-function Mutant GPIb fragment without Ristocetin. VWF:RCo, Ristocetin Cofactor activity: all assays that use platelets (formalin fixed or lyofilized) and Ristocetin.
Pearson’s r, Pearson correlation coefficient.
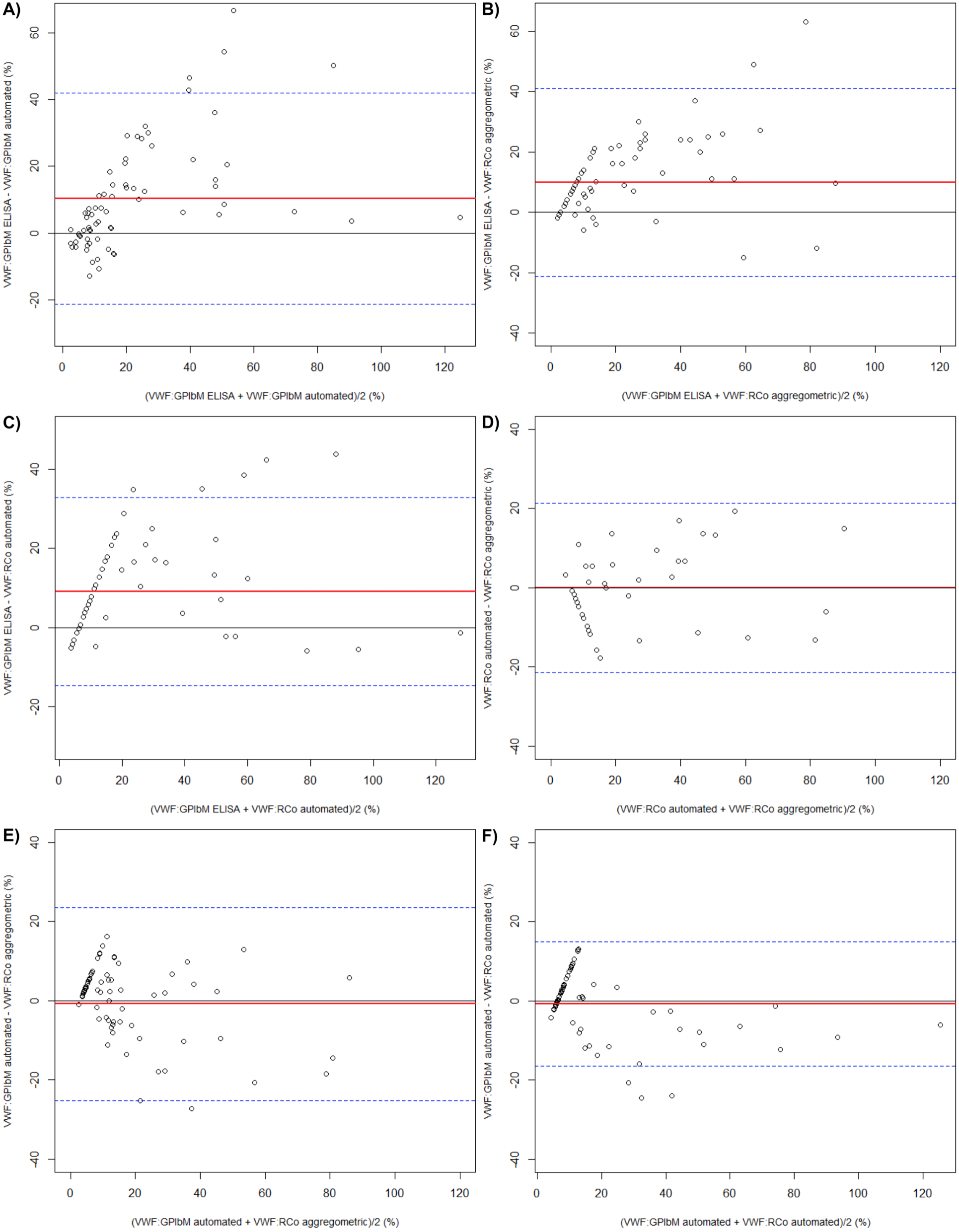
Figure 1. Bland-Altman plots. Red line = line of bias, black line = line of identity, dashed line = 95% limits of agreement.
CONCLUSIONS
Concordant results were obtained using the four platelet-dependent VWF activity assays in the evaluation of this group of type 2 VWD patients.
All identified outliers in this study were type 2B VWD patients with lack of HMWM, thus the eight type 2B New York (with HMWM) patients were not identified among this group.
The outliers showed higher ELISA VWF:GPIbM values than those obtained with automated VWF:GPIbM method.
Furthermore, when we compared the automated VWF:GPIbM assay with the automated VWF:RCo method, three outliers were identified. In these samples the VWF:GPIbM automated assay gave lower values than those obtained with the VWF:RCo assay.
REFERENCES
1. Favaloro EJ, Koutts J. Laboratory assays for von Willebrand factor: relative contribution to the diagnosis of von Willebrand’s disease. Pathology 1997; 29: 385-91.
2. Flood VH, Friedman KD, Gill JC, et al., Limitations of the ristocetin cofactor assay in measurement of von Willebrand factor function. J Thromb Haemost 2009; 7: 1832-9.
3. Stufano F, Baronciani L, Pagliari MT et al., Evaluation of an heterogeneous group of patients with von Willebrand disease using an assay alternative to ristocetin induced platelet agglutination. J Thromb Haemost. 2015 Oct; 13(10): 1806-14.
ACKNOWLEDGEMENTS
We acknowledge the illustration work of L.F. Ghilardini.