C. Valsecchi1, M. Mirabet2, I. Mancini3, L. Schiavone1, D. Mane-Padros2, F. Peyvandi1,3
1Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano, A. Bianchi Bonomi Hemophilia and Thrombosis Center and Fondazione Luigi Villa, Milan, Italy; 2Biokit Research & Development, Lliçà d’Amunt, Barcelona, Spain; 3Università degli Studi di Milano, Department of Pathophysiology and Transplantation, Milan, Italy
BACKGROUND
Thrombotic thrombocytopenic purpura (TTP) is a rare hematological disease characterized by the severe deficiency of ADAMTS13 activity. In acquired TTP, reduced ADAMTS13 activity is caused by autoantibodies inhibiting ADAMTS13 function (i.e., ADAMTS13 inhibitors) and/or increasing ADAMTS13 clearance from the circulation.
ADAMTS13 activity assays can be used to detect and titrate ADAMTS13 inhibitors. The HemosIL® AcuStar ADAMTS13 Activity assay (Instrumentation Laboratory, Bedford, MA, USA) is a fully automated 2-step chemiluminescent immunoassay for the quantification of ADAMTS13 activity. The aim of the study was to evaluate the performance of the HemosIL AcuStar ADAMTS13 Activity assay in the assessment of ADAMTS13 inhibitors.
MATERIALS AND METHODS
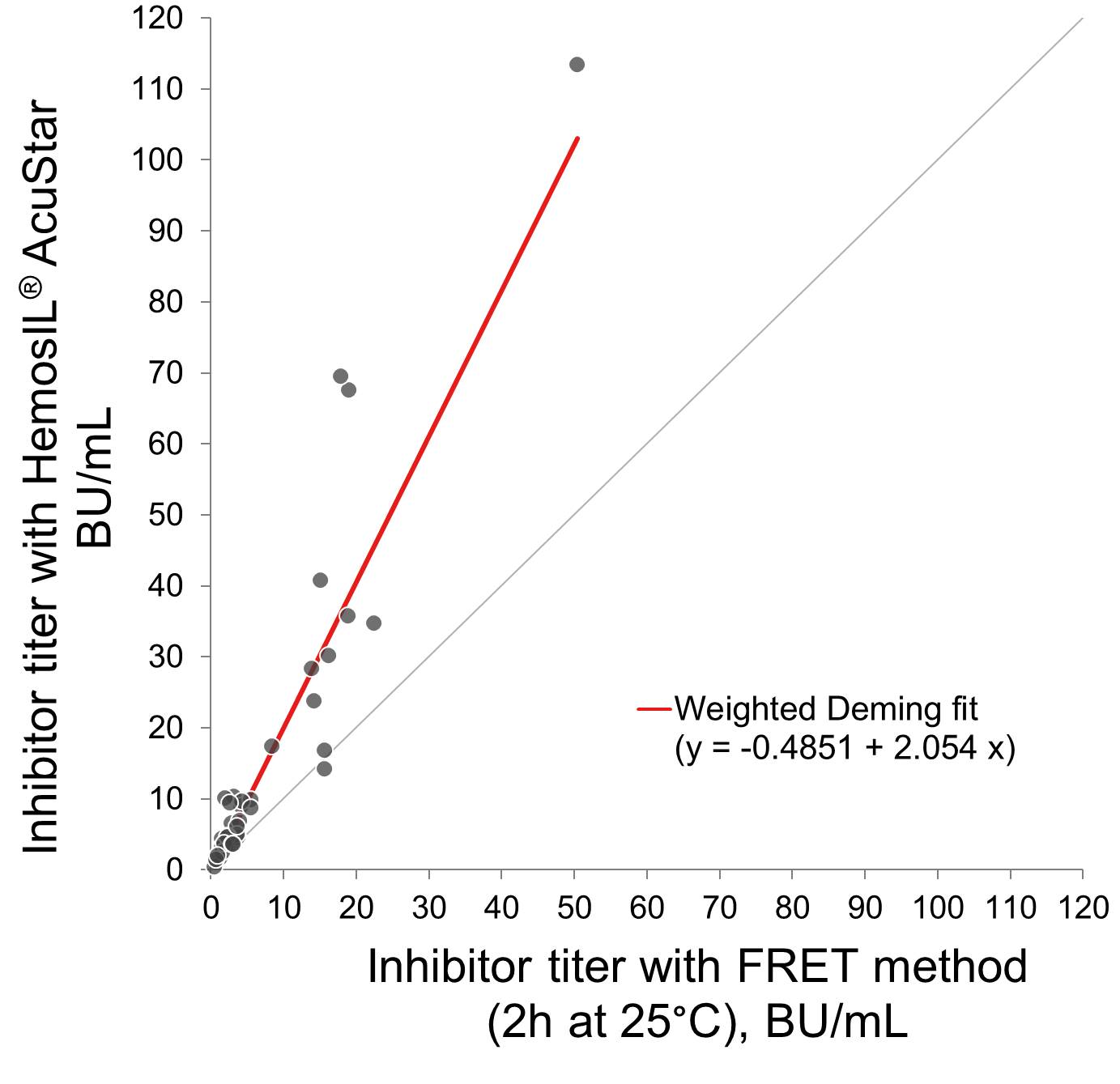
Thirty samples from normal donors and 50 from patients with acquired TTP, 23 in acute phase and 27 in remission (23/23 and 17/27 inhibitor positive using a reference method), were tested for the presence of anti-ADAMTS13 inhibitors using a mixing procedure. Heat-inactivated samples were mixed 1:1 with HemosIL Normal Control (pooled normal plasma) and incubated for 30 min at 37°C; activity of sample mixtures was measured with the HemosIL® AcuStar ADAMTS13 Activity assay (Figure 1). Results were compared with data obtained with a reference method using a similar mixing procedure (heat-inactivated plasma mixed 1:1 with pooled normal plasma), followed by incubation for 2 h at 25°C and measurement of the ADAMTS13 activity of the mixtures with an in-house FRETS-VWF73 assay (Mancini et al., JTH 2012;10:1439-42) (Figure 2). If required, HemosIL Factor Diluent or PBS + 4% BSA were used as sample diluent for the HemosIL AcuStar and the FRETS-VWF73 assay, respectively. Residual ADAMTS13 activity (100 x Activity of Patient Mixture / Activity of Control Mixture) was calculated. Values between 25% and 75% were used for calculation of the inhibitor titer, according to the Bethesda method.
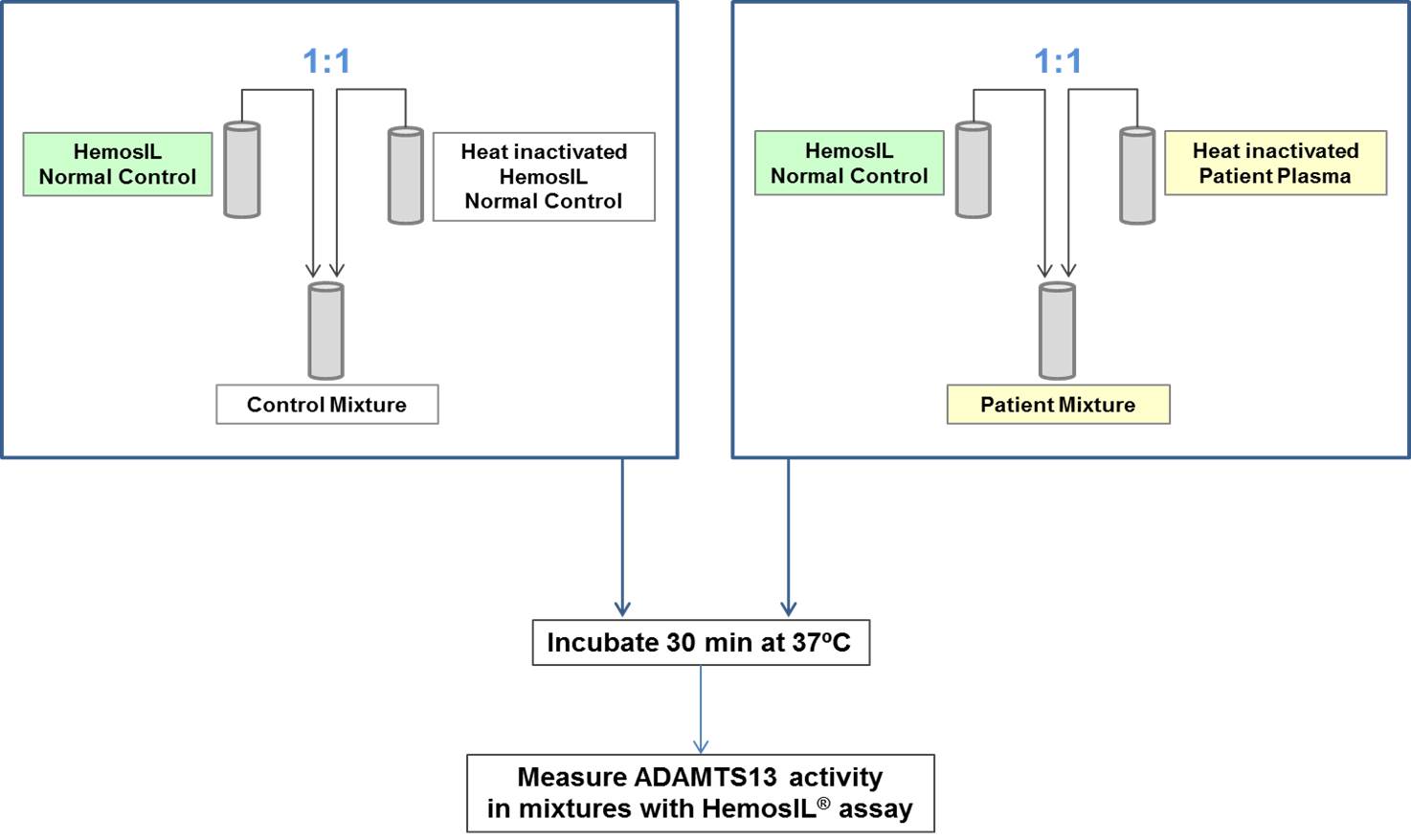
Figure 1. Assessment of ADAMTS13 inhibitors with the AcuStar ADAMTS13 Activity assay.
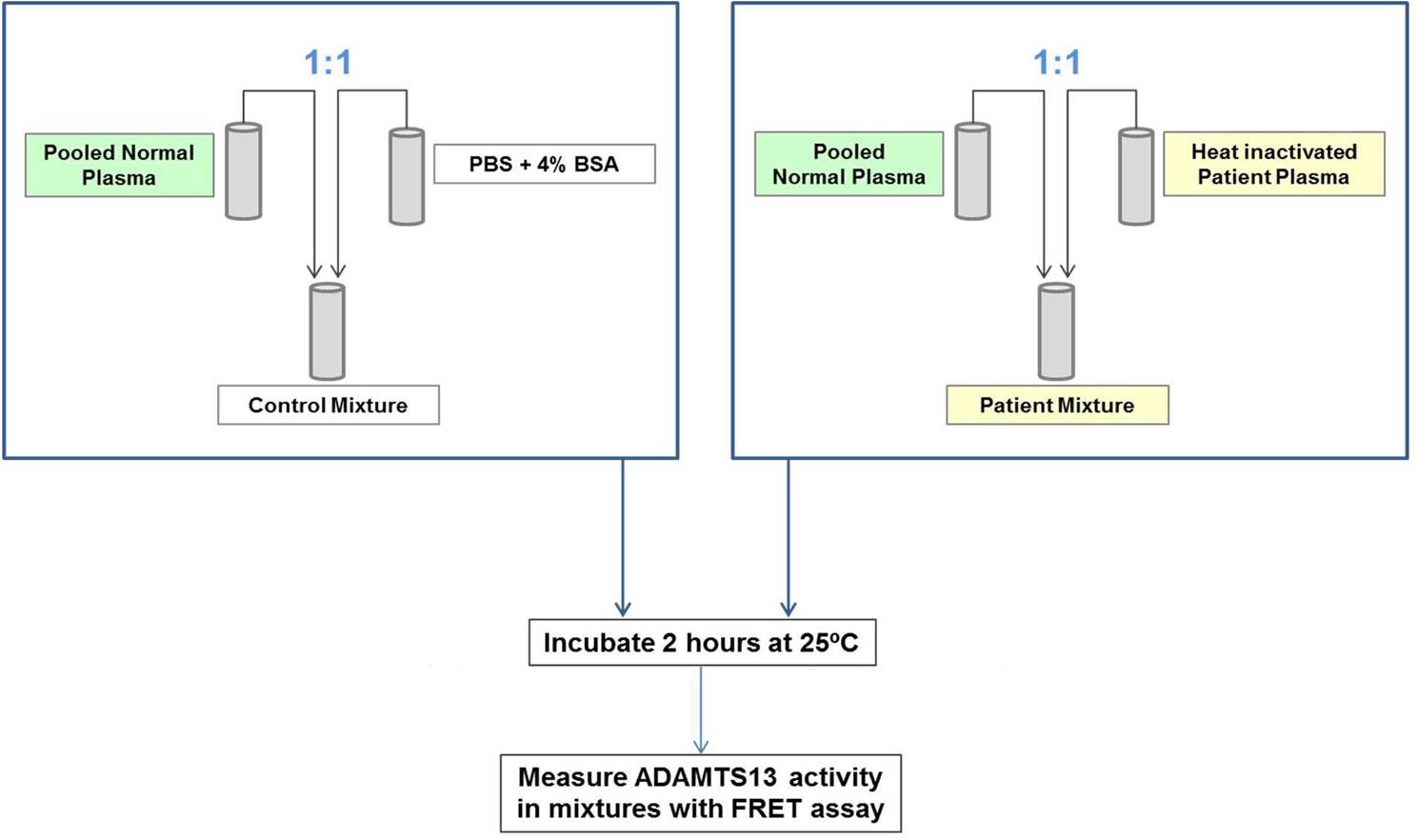
Figure 2. Assessment of ADAMTS13 inhibitors with the in-house FRETS-VWF73 assay.
RESULTS
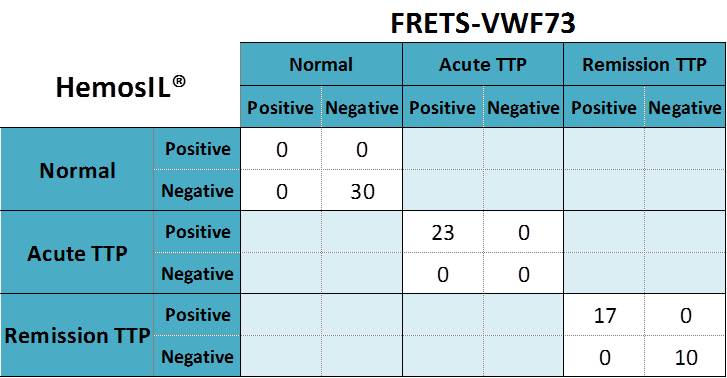
Qualitative comparison between the HemosIL® AcuStar ADAMTS13 Activity and FRETS-VWF73 methods
Full agreement between methods was observed regarding the detection of ADAMTS13 inhibitors.
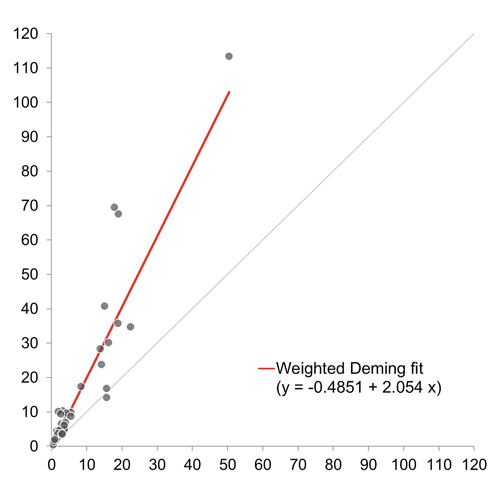
Quantitative comparison between the HemosIL® AcuStar ADAMTS13 Activity and FRETS-VWF73 methods
For the 40 samples with detectable inhibitors, the HemosIL and the reference FRETS-VWF73 assays showed good correlation (r = 0.93), although inhibitor titers measured by the HemosIL assay were, on average, 2-fold higher.
CONCLUSIONS
- The HemosIL AcuStar ADAMTS13 activity assay showed a good performance in identifying the presence of ADAMTS13 inhibitors in TTP patients, compared with the in-house FRETS-VWF73 reference method.
- The short time of incubation of the mixture allows for quick inhibitor results without affecting assay clinical sensitivity and specificity.
- Further investigation is needed to explore the reasons of the higher ADAMTS13 inhibitors titers measured using the HemosIL method compared with the in-house FRETS-VWF73 assay.