Christoph Königs1, Shannon Meeks2, Håkan Malmström3, Nisha Jain4, Stefan Lethagen3,5
1Department of Pediatrics and Adolescent Medicine, Frankfurt University Hospital, Frankfurt am Main, Germany; 2Aflac Cancer and Blood Disorders Center, Department of Pediatrics, Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA, USA; 3Swedish Orphan Biovitrum AB, Stockholm, Sweden; 4Sanofi, Waltham, MA, USA; 5Copenhagen University, Copenhagen, Denmark
Background
- Development of neutralising anti-factor VIII (FVIII) alloantibodies (inhibitors) is the most serious complication in patients with haemophilia A, affecting approximately 25–30% of patients with severe haemophilia A.1,2
- Immune tolerance induction (ITI), which consists of frequent and prolonged administration of high doses of FVIII, is the standard of care for the eradication of inhibitors and restoration of the clinical efficacy of FVIII replacement therapy.3,4
- Patients who have previously failed ITI are more likely to fail subsequent ITI attempts.5 Other risk factors for poor response to ITI include inhibitor titre ≥10 Bethesda units (BU) at ITI start, historic peak inhibitor titre >200 BU, age >7 years at ITI start, and >24 months between inhibitor diagnosis and ITI start.5
Study Rationale
- Preclinical data suggest that extended half-life recombinant FVIII Fc fusion protein (rFVIIIFc) has immunomodulatory properties.6
- Previously published data from a retrospective chart review suggest that a rapid time to tolerisation can be achieved with rFVIIIFc in subjects at high risk of ITI failure.7,8
- However, there is a need to describe the outcome of ITI using a standardised treatment protocol, especially in patients with a poor prognosis of ITI success.
- The verITI-8 study (NCT03093480) is evaluating the outcome of ITI with rFVIIIFc in patients with severe haemophilia A and inhibitors who are undergoing their first ITI treatment.
- This presentation reports on the ReITIrate study (NCT03103542), which is designed to prospectively evaluate the outcome of rescue ITI, within a timeframe of 60 weeks, using a standardised rFVIIIFc protocol in patients with severe haemophilia A who have failed previous ITI attempts, including the use of immunosuppressants.
Objective
- The objective of this presentation is to report baseline characteristics for patients enrolled in ReITIrate between November 2017 and December 2018.
Materials and Methods
Overview of Study Design
- ReITIrate is a prospective, interventional, multicentre, phase 4 open-label study conducted in Europe and North America to evaluate the outcome of rescue ITI with rFVIIIFc (Figure 1).
- After a 4- to 6-week screening period, all eligible patients will receive rFVIIIFc 200 IU/kg/day until successful tolerisation or for a maximum of 60 weeks (Figures 1 and 2).
- In patients who achieve ITI success, the rFVIIIFc dose will be gradually reduced to a prophylactic dose during a 16-week tapering period. Thereafter, patients will receive prophylactic rFVIIIFc during a 32-week follow-up period, with an additional 2 weeks of safety follow-up (Figures 1 and 2).
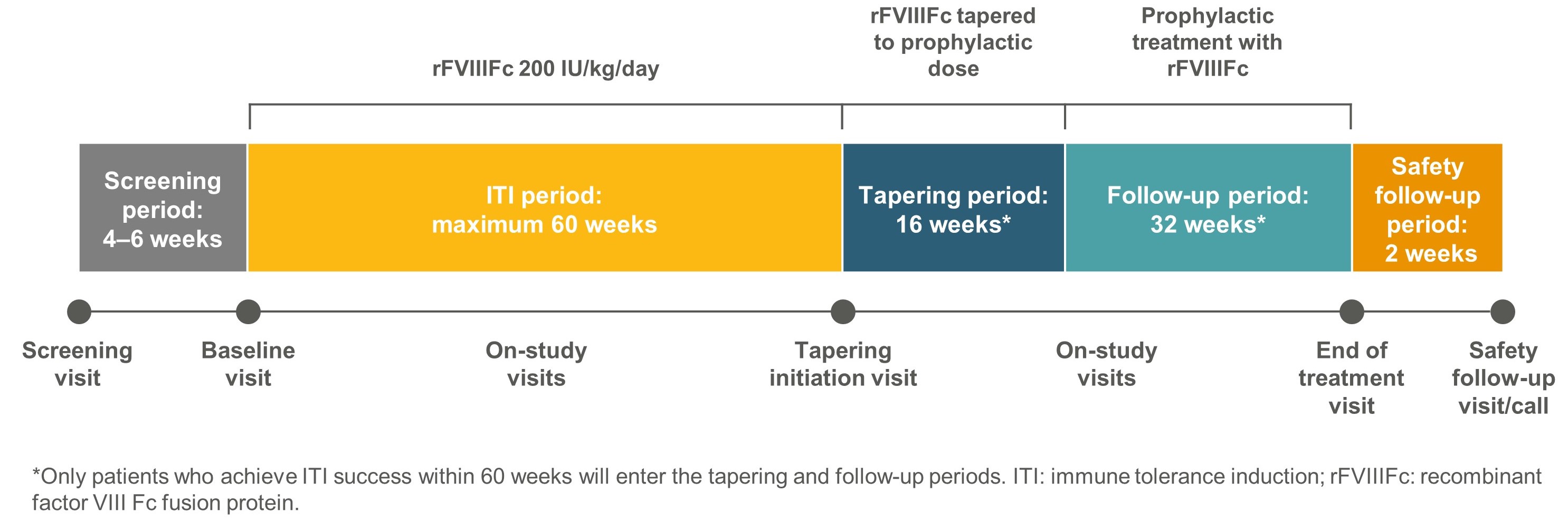
Figure 1. ReITIrate study design
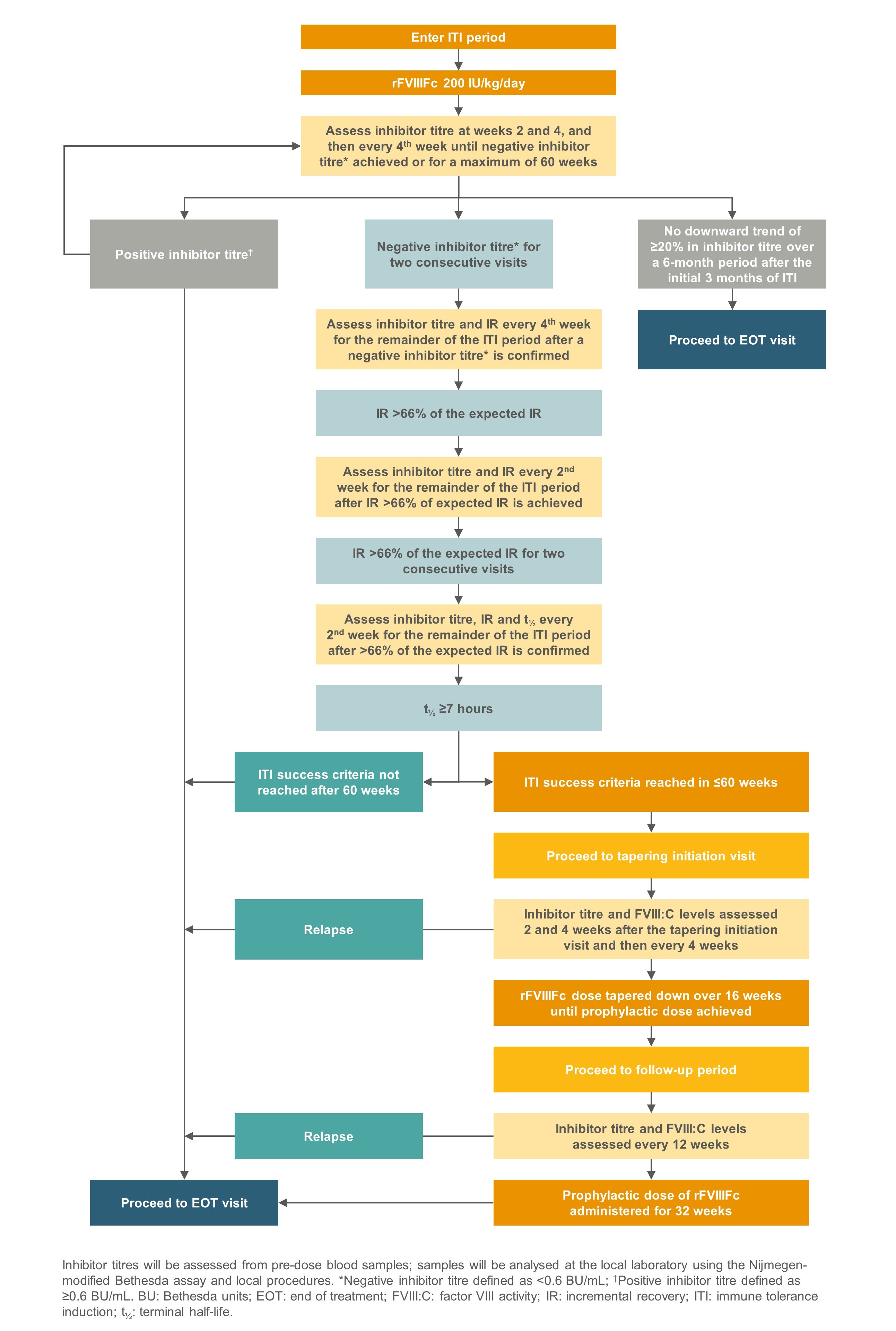
Figure 2. Assessment of ITI outcome in ReITIrate
ReITIrate Study Objectives and Primary Endpoint
- The primary objective is to describe the outcome of ITI performed with rFVIIIFc (200 IU/kg/day) within a timeframe of 60 weeks, in patients who have failed previous ITI (Table 1).
- The primary endpoint is ITI success, defined as achieving all of the following :
- Negative inhibitor titre (<0.6 BU/mL by the Nijmegen-modified Bethesda assay) at two consecutive visits
- FVIII incremental recovery >66% of expected incremental recovery at two consecutive visits
- FVIII terminal half-life of ≥7 hours
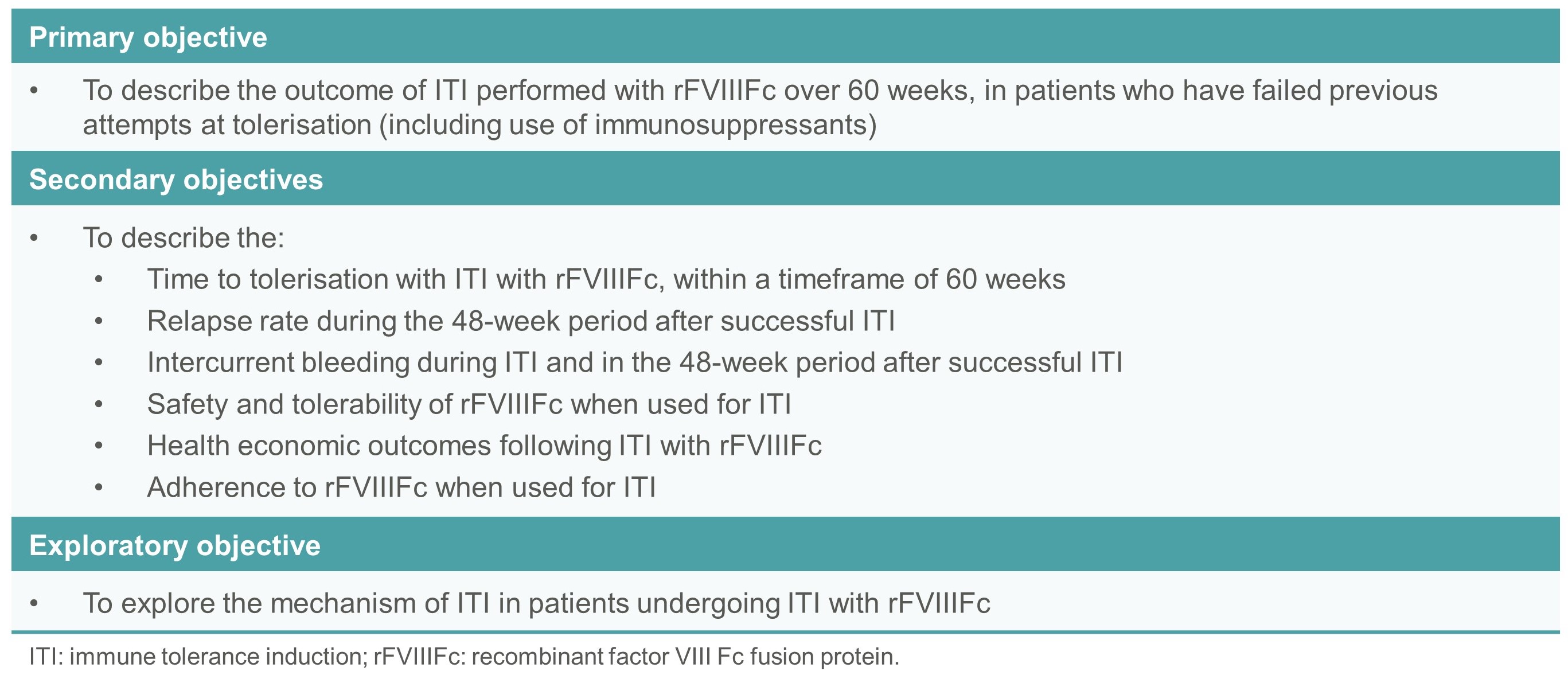
Table 1. Study objectives
Patient Eligibility
- The ReITIrate study enrolled severe haemophilia A patients with inhibitors who had failed previous ITI attempts. Key patient eligibility criteria are shown in Table 2.
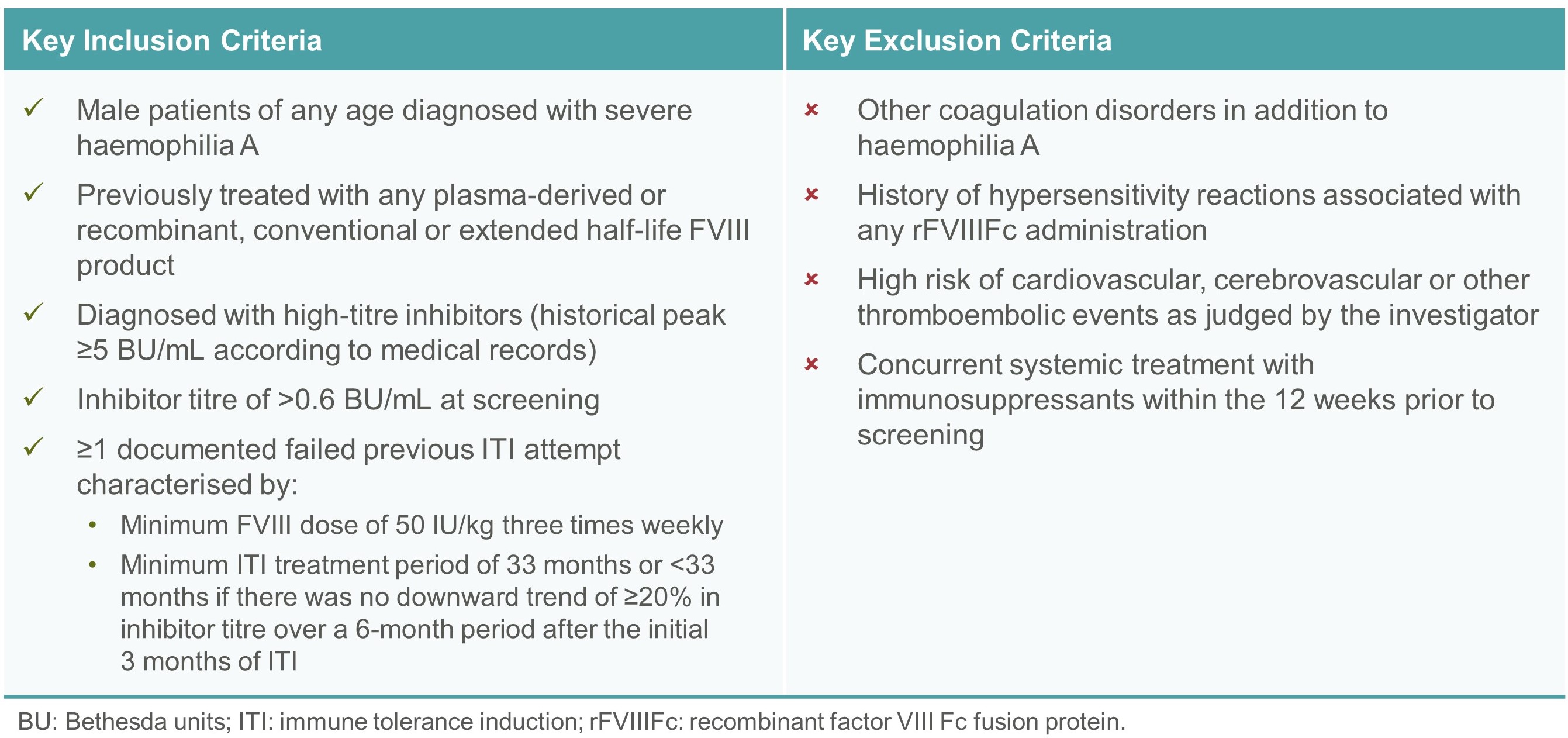
Table 2. Patient eligibility criteria
Statistical Analysis
- Baseline characteristics for patients enrolled between November 2017 and December 2018 are reported here using descriptive statistics and listings.
spacer
Results
Patients
- Sixteen subjects were included in the study between November 2017 and December 2018 (Tables 3 and 4).
- The median (range) age at study enrolment was 7.5 (2–46) years. The median (range) number of prior ITI attempts was 1 (1–3) and the total median (range) ITI duration was 51.5 (12–155) months (Tables 3 and 4).
- The median (range) inhibitor titre at screening was 11 (1–635) BU/mL and the median (range) historical peak was 125 (10–3000) BU/mL (Tables 3 and 4).
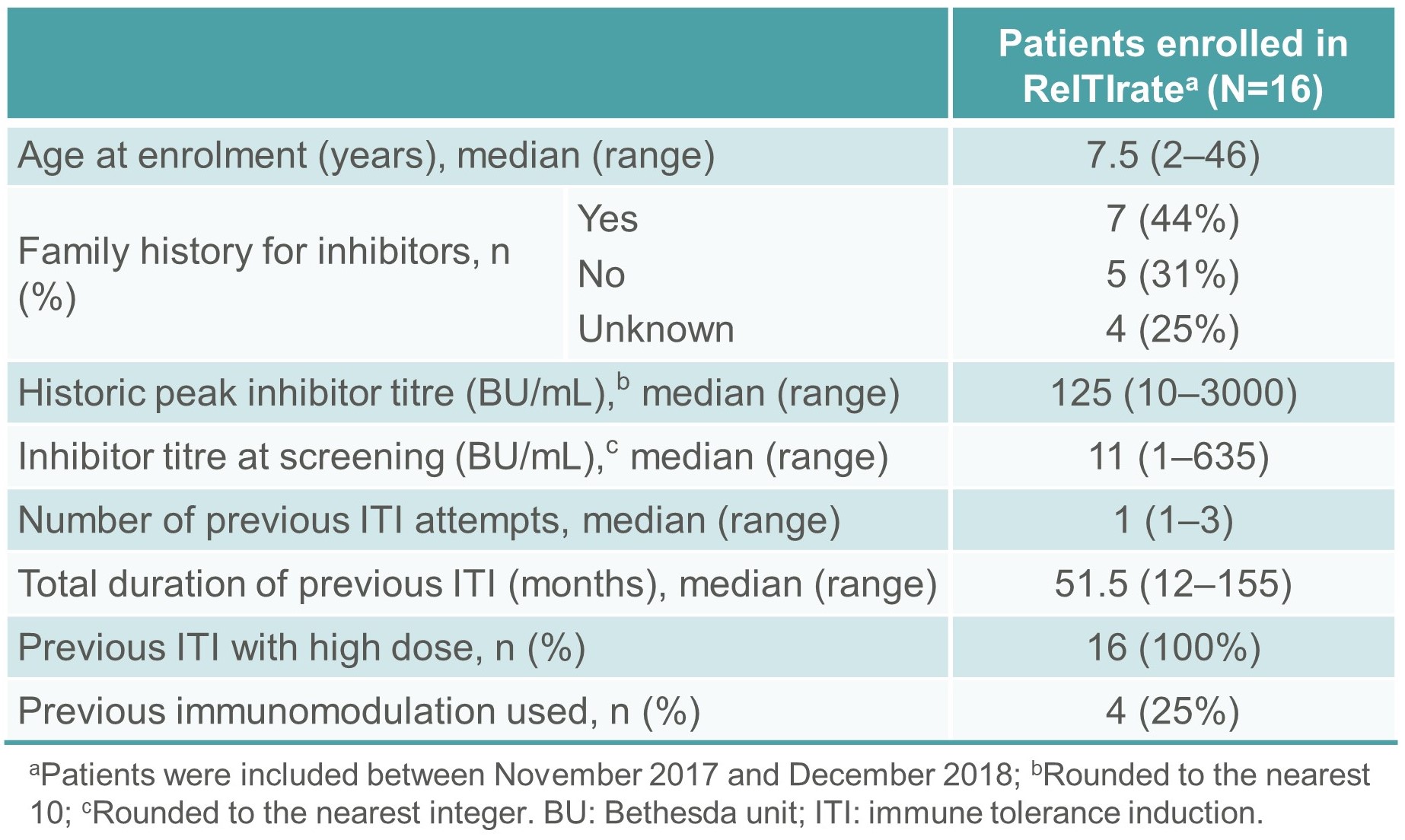
Table 3. Summary demographics and baseline characteristics of patients enrolled in ReITIrate
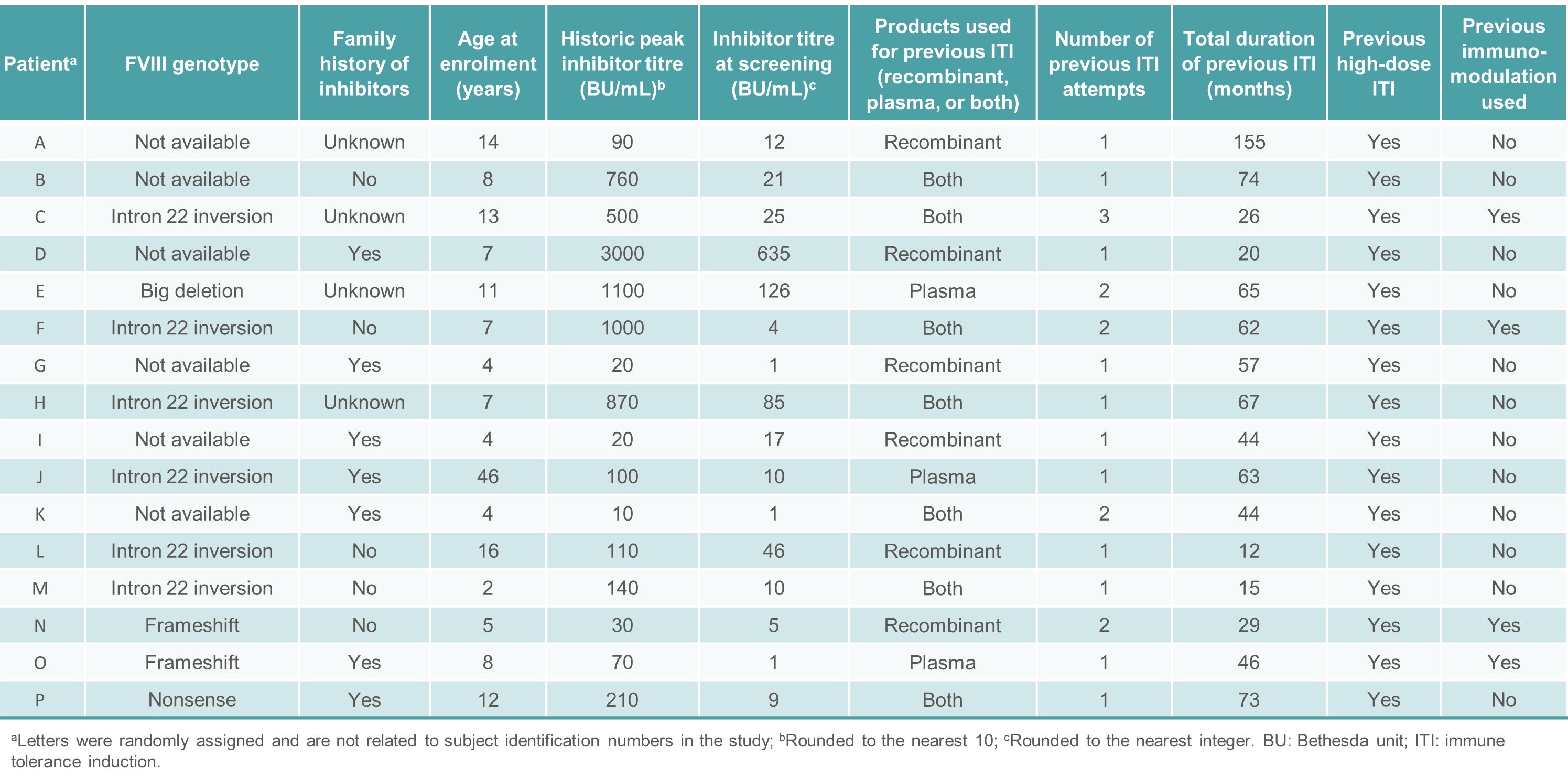
Table 4. Individual baseline characteristics of patients enrolled in ReITIrate between November 2017 and December 2018
Treatment of Bleeds and Prophylaxis with Bypassing Agents Prior to Enrolment
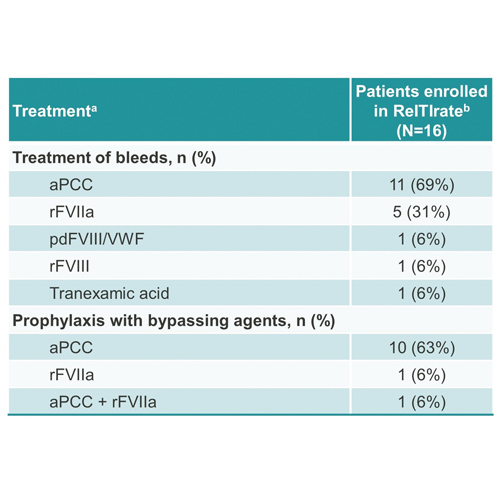
- During the 12 months prior to enrolment in ReITIrate, the median (range) number of bleeds was 5 (0–24). The products used for the treatment of bleeds are shown in Table 5.
- Twelve subjects received prophylaxis with bypassing agents during this period (Table 5).
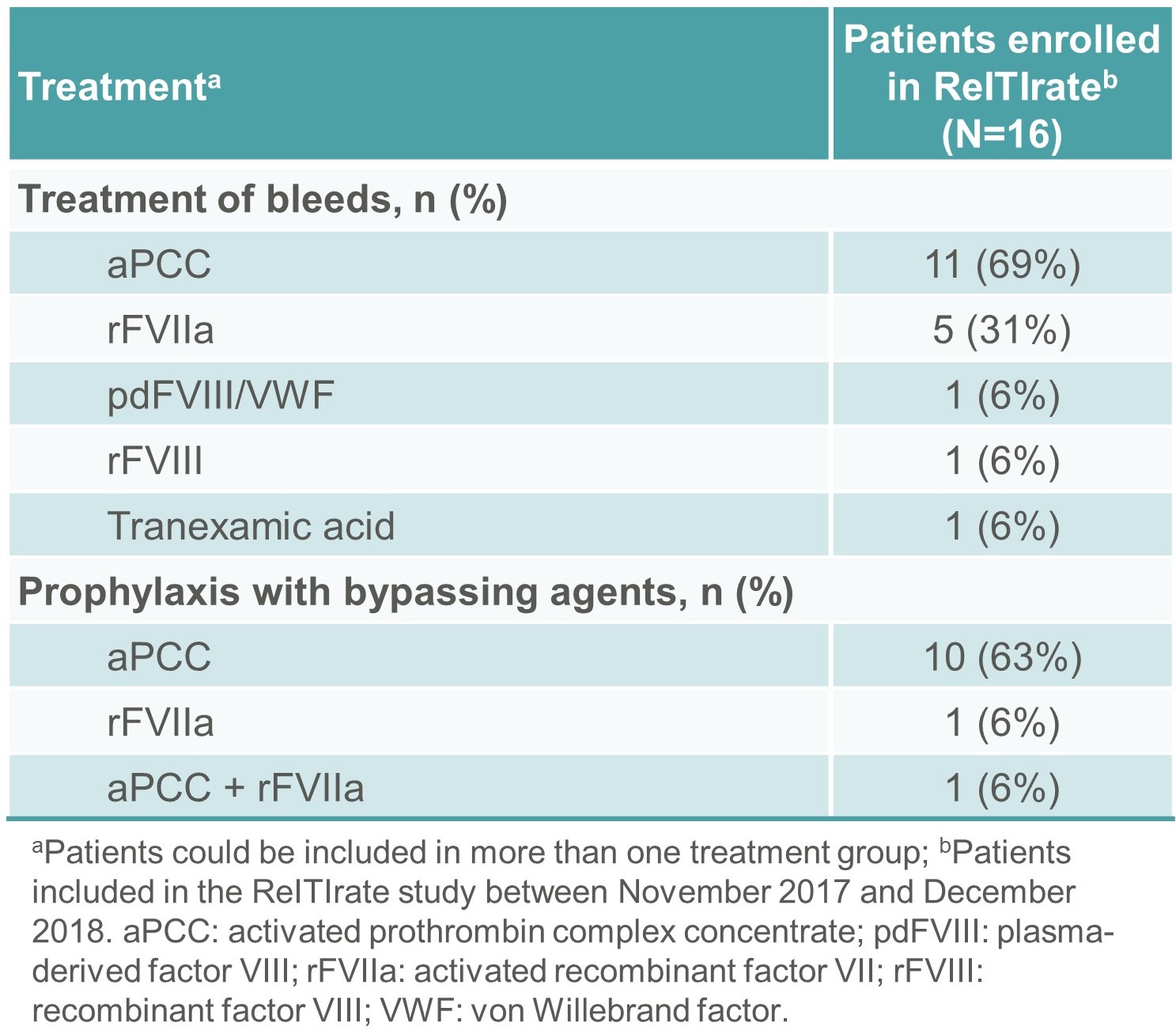
Table 5. Treatment of bleeds and prophylaxis with bypassing agents in the 12 months prior to enrolment in ReITIrate
Conclusions
- This is the first prospective study describing rescue ITI with an extended half-life rFVIII product.
- Enrolled subjects have multiple risk factors for poor ITI outcome and long duration of previous ITI.
- There is an unmet need for successful tolerisation in such patients, allowing regular FVIII prophylaxis and potentially leading to improved clinical outcomes and quality of life.
References
1. van den Berg HM. Thromb J 2016;14:31;
2. Peyvandi F. et al. Lancet 2016;388:187–197;
3. DiMichele DM. et al. Haemophilia 2007;13:1–22;
4. Brackmann HH. et al. Haemophilia 2018;24:3–14;
5. Oldenburg J. et al. Haemophilia 2014;20:83–91;
6. Krishnamoorthy S. et al. Cell Immunol 2016;301:30–39;
7. Carcao M. et al. Haemophilia 2018;24:245–252;
8. Carcao M. et al. Blood 2018;132:2500.
Disclosures
This research was funded by Sobi and Sanofi. Sobi and Sanofi reviewed and provided feedback on the e-poster. The authors had full editorial control of the e-poster and provided their final approval of all content.
C. Königs: Institutional research support from Bayer, Biotest, Bioverativ, CSL Behring, Grifols, Intersero, Jansen, Novo Nordisk, Pfizer, Roche/Chugai, Sobi, Shire/Takeda; Consultancy for Bayer, Bioverativ, CSL Behring, Grifols, Roche/Chugai, Sobi, Shire/Takeda; Honoraria/Speakers Bureau for BFSH, CSL Behring, Grifols, MSD, Novo Nordisk, Pfizer, Roche/Chugai, Shire/Takeda, Sobi. S. Meeks: Consultancy for Bayer, Bioverativ/Sanofi, Sobi, CSL Behring, Grifols, Genentech, Novo Nordisk, HEMA Biologics, Shire/Takeda. H. Malmström: Shareholder and employee of Sobi. N. Jain: Employee of Sanofi. S. Lethagen: Shareholder and employee of Sobi.
Acknowledgements
We thank the patients and investigators who are involved in the ReITIrate study. Editorial assistance for the development of this e-poster was provided by Jessica Patel, PhD, and Ricardo Milho, PhD, Costello Medical, UK, and was funded by Sobi.