Dekimpe Charlotte1, Roose Elien1, Dewaele Aurélie1, Horta Sara1, Tersteeg Claudia1, Pareyn Inge1, Vandenbulcke Aline1, Deckmyn Hans1, Feys Hendrik B.2, Tellier Edwige3, Kaplanski Gilles3, Coppo Paul4, De Meyer Simon F.1, Veyradier Agnès5, Vanhoorelbeke Karen1
1Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Kortrijk, Belgium; 2Transfusion Research Center, Belgian Red Cross-Flanders, Ghent, Belgium; 3INSERM, Aix-Marseille Université, Vascular Research Center of Marseille, Marseille, France; 4Departement d’hématologie Clinique, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris and Université Pierre et Marie Curie, Paris, France; 5Service d’hématologie biologique, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris and EA3518, Institut Universitaire d’Hématologie, Hôpital Saint Louis, Université Paris Diderot, Paris, France
Introduction and Aim
Thrombotic thrombocytopenic purpura (TTP) is a rare, life-threatening microangiopathic disorder, characterized by severe thrombocytopenia, hemolytic anemia and organ ischemia, which is caused by a functional deficiency in the von Willebrand factor (VWF)-cleaving protease ADAMTS13. ADAMTS13 is a multi-domain metalloprotease consisting of a metalloprotease (M), disintegrin-like (D), cysteine-rich (C), spacer (S), 8 thrombospondin type 1 repeats (T1-8) and 2 CUB domains (Fig.1). Immune-mediated TTP (iTTP) is characterized by the presence of inhibitory and/or non-inhibitory anti-ADAMTS13 autoantibodies. ADAMTS13 antigen (Ag) levels are moderately to severely reduced in most iTTP patients, indicating that antibody-mediated clearance is the major pathogenic mechanism in this disease. Recently, it has been shown that severely reduced ADAMTS13 Ag levels are associated with a higher mortality rate in iTTP, indicating the prognostic value of ADAMTS13 Ag determination in these patients.
In the current study, we measured ADAMTS13 Ag levels in a large cohort of healthy donors and iTTP patients using our in-house developed monoclonal antibody (mAb)-based ADAMTS13 Ag ELISA (Fig.1) (1), we validated this ELISA in terms of sensitivity and reproducibility (2) and investigated whether anti-ADAMTS13 antibodies present in plasma of iTTP patients influence correct determination of ADAMTS13 Ag levels using this in-house developed ADAMTS13 Ag ELISA (3).

Figure 1: Epitope overview of the assay monoclonal antibodies 3H9, 17G2 and 19H4. Representation of the domain structure of ADAMTS13 with epitope overview of the assay mAbs (3H9, 17G2 and 19H4). In our in-house developed ADAMTS13 Ag ELISA, ADAMTS13 is captured on 3H9 (5 µg/mL) and bound ADAMTS13 is detected using a mixture of biotinylated 17G2 and 19H4 (each 1.5 µg/mL) followed by HRP-labeled streptavidin.
Methods and Results
(1) ADAMTS13 antigen levels in a large cohort of healthy donors and iTTP patients
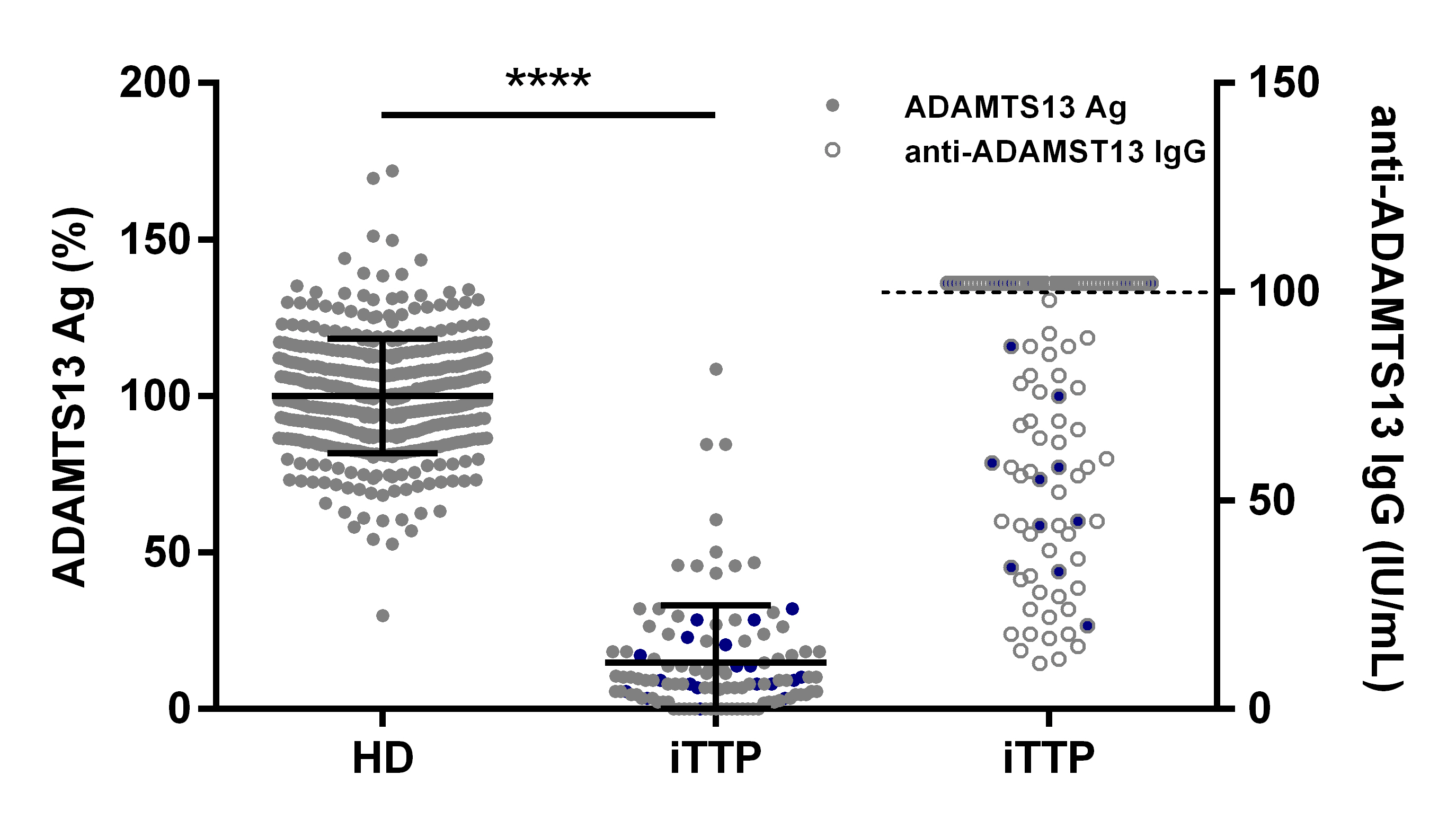
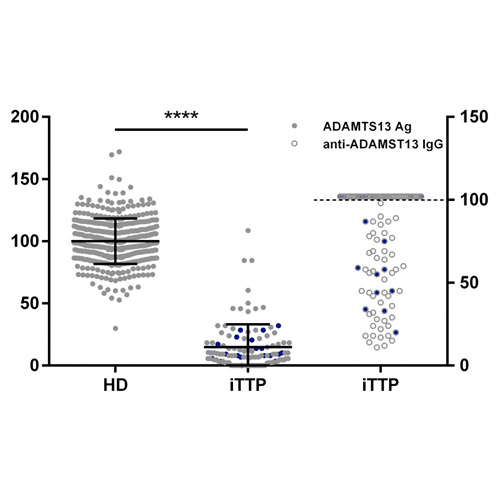
Figure 2: ADAMTS13 Ag levels are moderately to severely reduced in the majority of iTTP patients.
ADAMTS13 Ag levels were determined in 424 healthy donor (100 ± 18.22 %) and 112 acute iTTP (14.88 ± 18.27 %) plasma samples using our in-house developed ADAMTS13 antigen ELISA (●). Anti-ADAMTS13 autoantibody levels in iTTP plasma samples were measured using the Technozym ADAMTS13-inhibitor kit (○), with the upper limit of detection represented by the dotted line. Nineteen iTTP plasma samples (●) were selected for purification of total IgGs.
(2) Performance characteristics of the in-house developed ADAMTS13 Ag ELISA
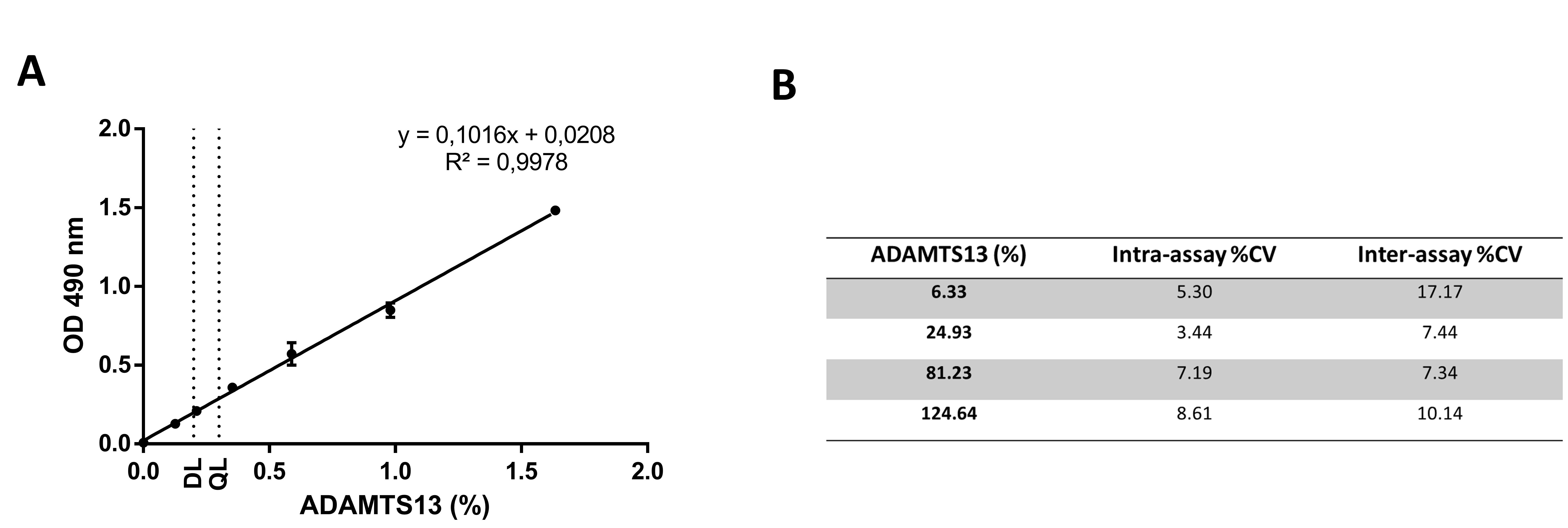
Figure 3: ADAMTS13 antigen assay performance characteristics.
(A) Representative reference curve to calculate unknown ADAMTS13 Ag levels with indication of the detection limit (DL) and quantification limit (QL).The DL and QL were determined by performing repeats of an ADAMTS13 deficient plasma sample. The DL (3xSD above the mean of blank sample) was 0.20% (2.53% when taking plasma dilution into account), the QL (10xSD above the mean of blank sample) was 0.30% (3.72% when taking plasma dilution into account). (B) The intra-assay %CV was determined using six replicates of four QC samples in one run. The inter-assay %CV was determined by testing these four QC samples on six replicates over different days.
(3) Influence of anti-ADAMTS13 autoantibodies on ADAMTS13 antigen determination

Figure 4: Anti-ADAMTS13 autoantibodies of iTTP patients do not hamper plasma ADAMTS13 Ag level determination using our in-house developed ELISA.
(A) Binding of purified total IgGs from iTTP patient plasma and from NHP to recombinant (r)ADAMTS13. Normalized OD490nm are depicted. (B) The effect of purified total IgGs from iTTP patient plasma on ADAMTS13 antigen determination. NHP (final dilution 1/100) was pre-incubated with purified IgGs of NHP (grey bar) or iTTP patients (final concentration 200 µg/mL) (blue bars) after which ADAMTS13 antigen levels where measured using our in-house developed ADAMTS13 antigen ELISA (n=3).
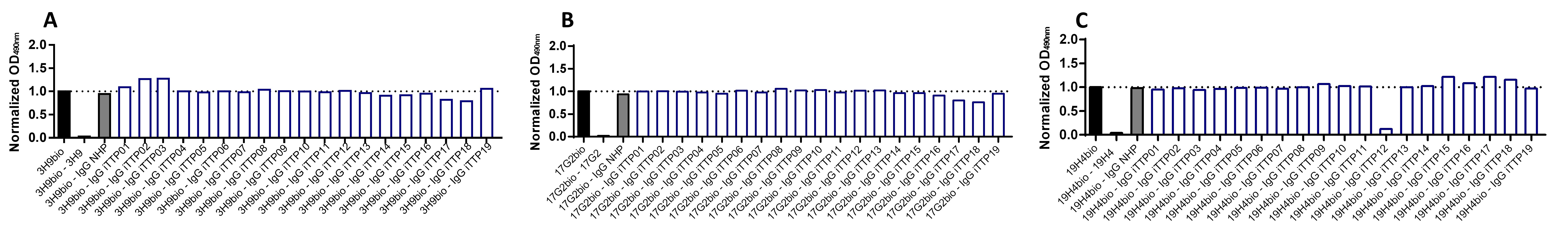
Figure 5: Competition between assay antibodies (3H9, 17G2, 19H4) and purified iTTP IgGs.
(A) Competition between 3H9bio and iTTP IgGs for binding to coated rADAMTS13. iTTP IgGs (iTTP01-19), NHP IgGs (400 µg/mL; final concentration 200 µg/mL) or 3H9 (10 µg/mL; final concentration 5 µg/mL) were pre-incubated with coated rADAMTS13 after which 3H9bio (final concentration at EC50) was added. Bound 3H9bio was detected with HRP-labeled streptavidin. Normalized OD490nm values are depicted. None of the iTTP IgGs competed with 3H9bio for rADAMTS13 binding. (B) Competition between 17G2bio and iTTP IgGs for binding to coated rADAMTS13. None of the iTTP IgGs competed with 17G2bio for rADAMTS13 binding. (C) Competition between 19H4bio and iTTP IgGs for binding to coated rADAMTS13. Only one iTTP IgG sample (iTTP12) competed with 19H4bio for rADAMTS13 binding.
Conclusions
The presence of anti-ADAMTS13 autoantibodies in plasma of iTTP patients does not influence ADAMTS13 antigen determinations using our in-house ELISA. The latter is thus a powerful tool to correctly determine ADAMTS13 Ag levels in iTTP patients. These data support routine measurements of ADAMTS13 antigen levels in these patients to have a better insight in disease prognosis.