Novembrino Cristina1, Boscolo Anzoletti Massimo1, Rossi Federica1, Mancuso Maria Elisa1, Peyvandi Flora1
1Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Milan, Italy
BACKGROUND and AIM
Measurement of Factor IX is required for quantification of residual plasma FIX levels with a diagnostic purpose (Haemophilia B) or for performing recovery studies during treatment by replacement therapies. The one-stage clotting assay is the most used in routine laboratories; there are only two chromogenic assays commercially available at present. We aim to evaluate the analytical performance of the Biophen FIX chromogenic assay on the Sysmex CS-2400 analyzer.
METHODS
PATIENTS
- 40 with haemophilia B (HB severe, moderate and mild),
- 120 healthy Italian subjects (controls aged 18-69 years).
The clotting tests were performed on citrate platelet poor plasmas stored at -80°C.
The results obtained with the chromogenic assay BIOPHEN Factor IX (HYPHEN BioMed, France) were compared with the one-stage assays (using Actin FS and FIX deficient plasma; Siemens, Germany). All the tests were performed using Sysmex CS 2400 analyzer (Sysmex, Kobe, Japan).
Analytical performance and method comparison analysis were performed according to the Clinical & Laboratory Standards Institute (CLSI) guidelines.
RESULTS
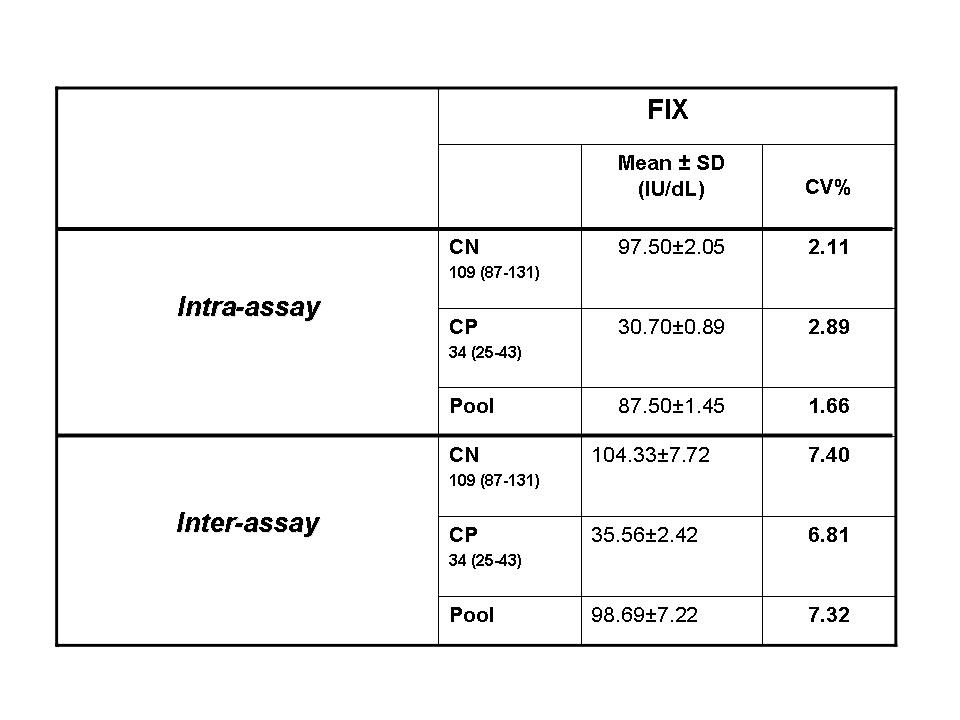
Imprecision analysis results are reported in Tables 1.
Linearity was good up to 1/128 (r=0.99);
mean recovery was 97.28%.
Analytical sensitivity was 0.2 IU/dL.
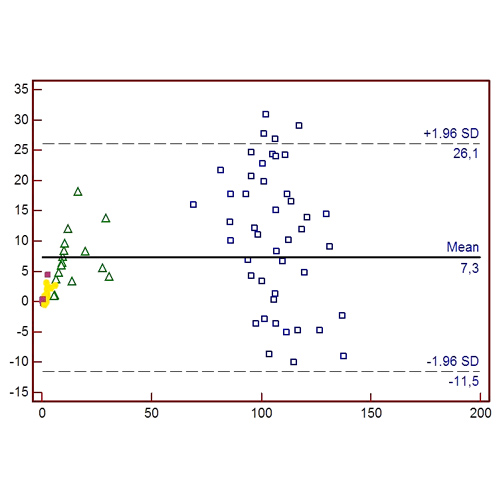
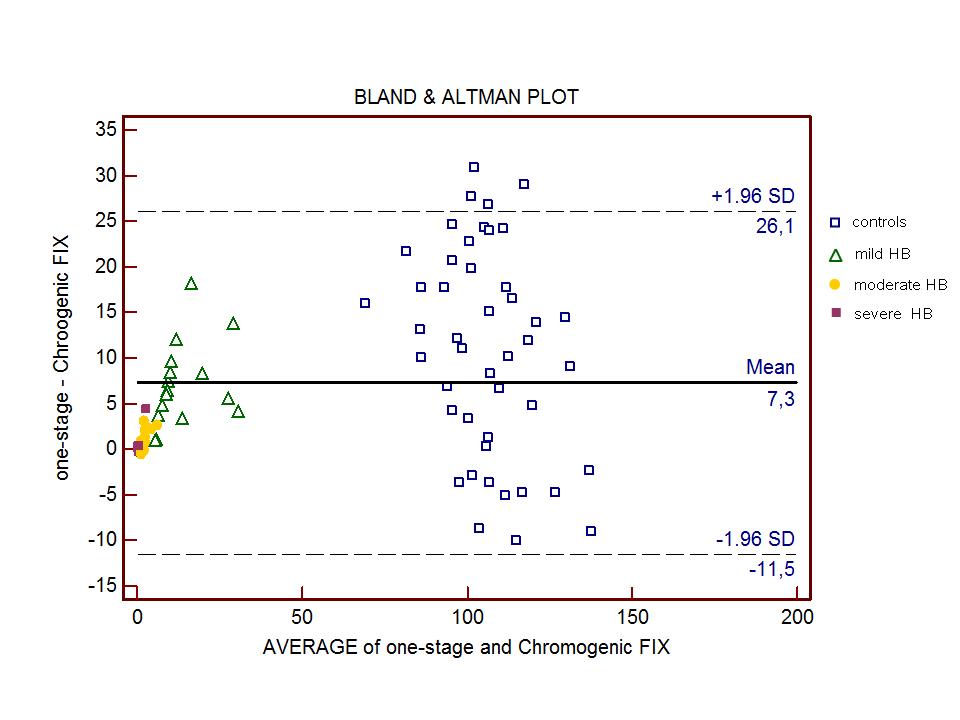
The reference range calculated on the 120 healthy subjects was 60 to 138% (95% Reference interval, Double-sided). Biophen FIX:C assay showed a good correlation with the one-stage assay (Spearmen’s Rank correlation 0.94) as shown also by the Bland and Altman Plot (Figure 1).
The diagnostic agreement between Biophen FIX:C and one-stage assay was satisfactory (K Cohen coefficient = 0.83); The K coefficient was 0.89 when Biophen FIX:C was compared with the historical classification of the patients, demonstrating an optimal diagnostic accuracy in HB patients.
CONCLUSIONS
Our results showed a good analytical performance of Biophen FIX:C as a suitable Chromogenic FIX functional assay. The introduction of chromogenic FIX test in addition to standard one-stage assay is important for the diagnosis and monitoring of therapeutic effects particularly in haemophilic patients treated with extended half-life products.
References
AdcocK DM. et all., Int J Lab Hematol 2018, 40: 621-9
Bowyer AE. et all., Haemophilia 2018, 24: 578-83
Kitchen S. et all. Haemophilia 2014, 20: 36-42
Dodt J. et all., Haemophilia 2015, 21: 543-9