Carmen Escuriola Ettingshausen1, Robert F. Sidonio, Jr.2, Johannes Oldenburg3, Behnaz Pezeshkpoor3, Jens Müller3, Barbara A. Konkle4, Shannon L. Meeks2, Yesim Dargaud5, Anna Pavlova3, Guy Young6, Jess Snedeker7
1HZRM Hämophilie Zentrum Rhein Main GmbH, Mörfelden-Walldorf, Germany; 2Aflac Cancer and Blood Disorders Center, Emory University, Atlanta, GA, USA; 3University Clinic Bonn, Institute of Experimental Haematology and Transfusion Medicine, Bonn, Germany; 4Bloodworks Northwest, Seattle, WA, USA; 5Unité d’Hémostase Clinique, Hôpital Cardiologique Louis Pradel, Bron, France; 6Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA, USA; 7Balgrist University Hospital, University of Zurich, Zurich, Switzerland
Background
- Immune tolerance induction (ITI) is the only clinically proven strategy for eradication of anti-factor VIII (FVIII) alloantibodies (inhibitors) and is recommended as the primary treatment option in European and US guidelines1-3
- The bispecific FIX/FX monoclonal antibody emicizumab is approved for bleeding prevention in people with haemophilia A and inhibitors, offering an alternative non-FVIII approach to the treatment of these patients
- FVIII replacement therapy and emicizumab have different mechanisms of action (Figure 1)4,5
Objectives
- The MOTIVATE study is seeking to answer the following questions:
- Will concomitant use of FVIII and emicizumab lead to improved rates of ITI success and shorter time to ITI success?
- Will concomitant use of FVIII and emicizumab provide improved bleeding protection in patients with inhibitors than either approach alone?
- What it the impact of FVIII dose on efficacy and safety?
- Will concomitant use of FVIII and emicizumab be safe and well tolerated?
- Will emicizumab prophylaxis and combination with ITI have an impact on risk of venous thrombosis?
- What is the impact of ITI, emicizumab prophylaxis, and their combination on markers of joint and bone health?
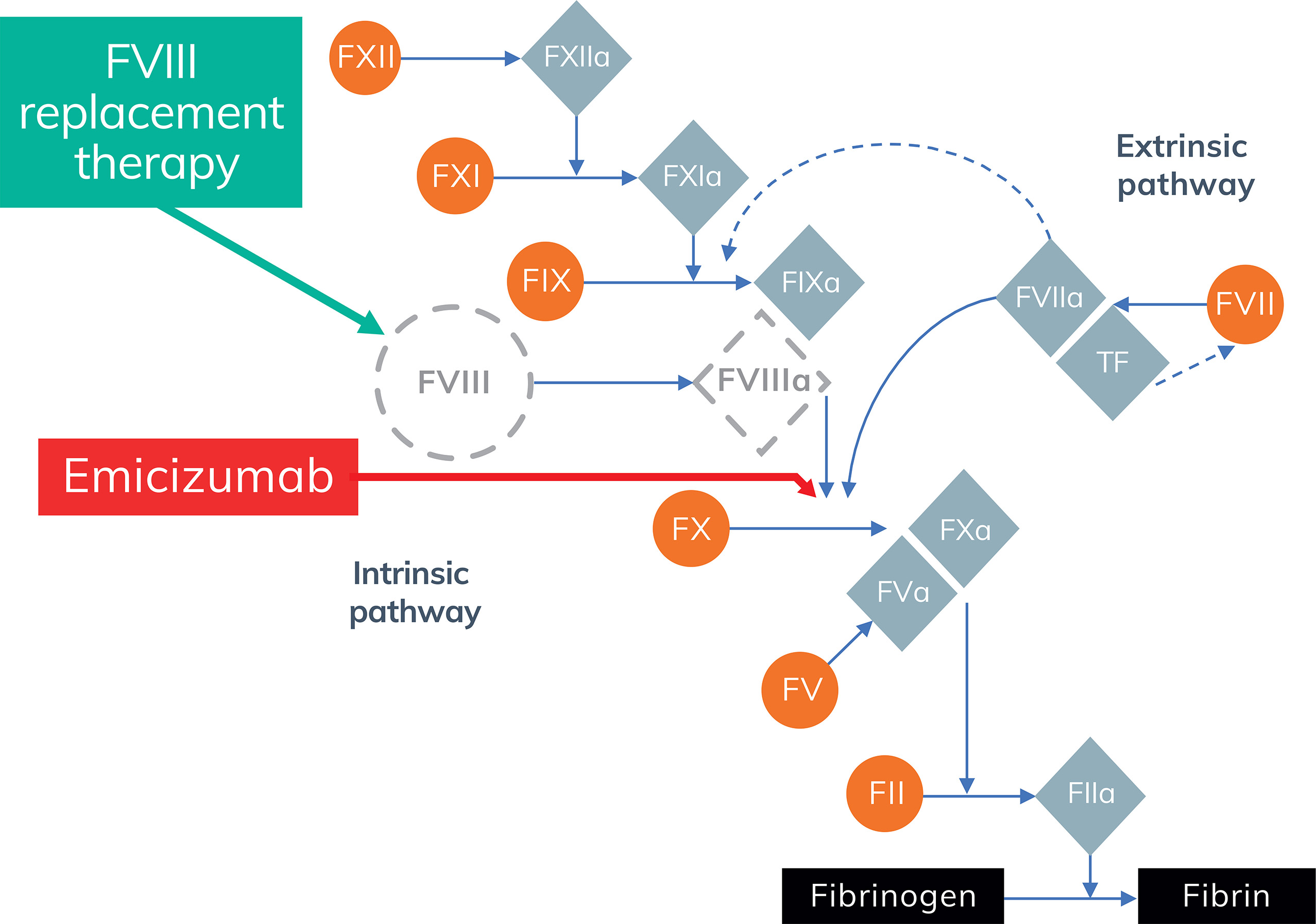
Figure 1. Coagulation pathway in haemophilia A (adapted from Perzborn et al.4)
Study design
- Investigator-initiated, prospective (and partly retrospective), observational, international study
- Study observation period: 5 years for each patient
- Planned enrollment: 120 patients at ~30 centres
- Inclusion criteria
- Male patients (any age) with haemophilia A of any severity and historical inhibitor titre ≥ 0.6 BU/mL, including those who have failed previous ITI attempt(s)
- Patients undergoing ITI with Nuwiq® (recombinant (r)FVIII, simoctocog alfa), octanate® (plasma-derived (pd)FVIII/VWF) or wilate® (pdVWF/FVIII) and/or receiving prophylactic therapy with emicizumab, aPCC or rFVIIa
- Exclusion criteria
- Coagulation disorder other than haemophilia A
- Data on three approaches to inhibitor management will be captured (Table 1)
- Choice of treatment will be at the discretion of the investigator and with consideration of the patient’s clinical condition and the prescribing information
- The potential risk for thrombotic events with emicizumab in combination with aPCC should be carefully considered
- Patients will record study information electronically using an eDiary (Figure 2)
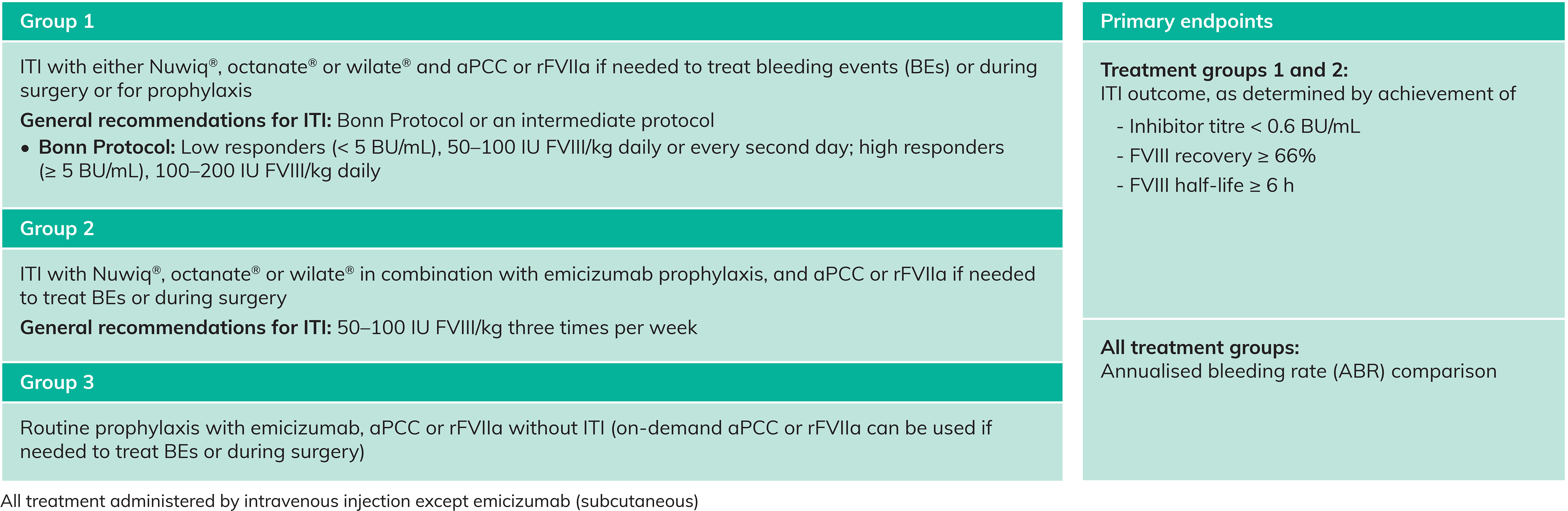
Table 1. Treatment groups and primary endpoints

Figure 2. eDiary documentation
Prinicipal Investigators and Steering Committee members are internationally recognised


Table 2. MOTIVATE Principal Investigators and Steering Committee
Optional sub-studies
- Optional sub-studies and their lead investigators are shown in Figure 3
- The Joint Health Markers MOTIVATE sub-study will compare the
role of FVIII vs. emicizumab in protecting joints, and aims to uncover new early degenerative markers- FVIII may have a role beyond haemostasis, in bone metabolism6-8 and haemophilic arthropathy9,10
- Although it is known that FVIII treatment protects joints10, the effect of emicizumab on joint health is not yet known
- In addition to well established joint health markers, this sub-study will include an exploratory proteomics analysis
- The Thrombotic Risk Assessment MOTIVATE sub-study will investigate the effect of inhibitor eradication strategies on coagulation parameters, including activated protein C (APC), von Willebrand factor (VWF) and D-dimer (Figure 4)
- Thrombotic microangiopathy (TMA) and thrombotic events have been reported in patients treated with emicizumab11
- Reduced sensitivity to APC, an important down-regulator of haemostatic function, is associated with an increased risk for venous thrombosis12,13
- By investigating the effect of emicizumab on APC-mediated downregulation, and other mediators of coagulation, this sub-study aims to improve the understanding of thrombotic pathways that may be influenced by emicizumab

Figure 3. MOTIVATE sub-study lead investigators
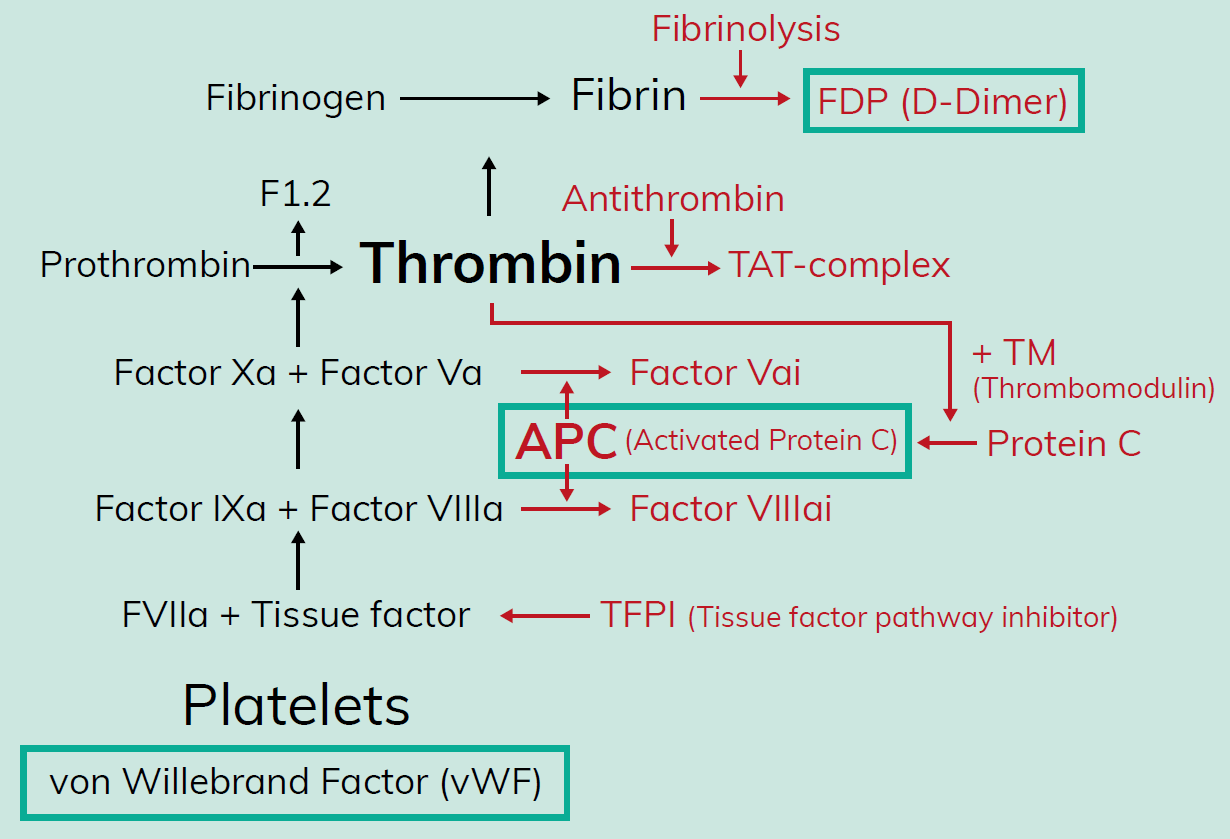
Figure 4. Thrombin-mediated coagulation
Conclusions
- MOTIVATE will collect real-world clinical experience of current practice and new approaches for the management of people with haemophilia A and inhibitors
- The study will help us to:
- Document and compare safety and efficacy of “standard of care” ITI vs. novel ITI approaches combining FVIII with emicizumab
- Document and compare safety and efficacy of ITI vs. emicizumab prophylaxis
- Evaluate and compare the impact of different treatment approaches on indicators of joint and bone health and risk for thrombosis
- Better understanding the different ITI approaches and options for managing people with haemophilia A and inhibitors will hopefully contribute to support decision making regarding the best choice of treatment
- For more information on the MOTIVATE study please contact motivate@syneoshealth.com
References
1. Giangrande PL et al. Orphanet J Rare Dis 2018; 13:66.
2. Valentino LA et al. Haemophilia 2015; 21:559-67.
3. Carcao M et al. Haemophilia 2019; 25:676-84.
4. Perzborn E et al. Nat Rev Drug Discov 2011; 10:61-75.
5. Lenting P et al. Blood 2017; 130:2463-8.
6. Kiper Unal HD et al. Am J Blood Res 2017; 7:59-66.
7. Kempton CL et al. Haemophilia 2015; 21:568-77.
8. Liel MS et al. Br J Haematol 2012; 158:140-3.
9. Muto A et al. Blood 2014; 124:3165-71.
10. Manco-Johnson M et al. N Engl J Med 2007; 357:535-44.
11. https://www.emicizumabinfo.com/content/dam/gene/emicizumabinfo/patient/pdfs/thrombotic-microangiopathy.pdf
12. de Visser MC et al. Blood 1999; 93:1271-6.
13. Svensson PJ & Dahlbäck B. N Engl J Med 1994; 330:517-22.
