Emilio Musi1, Martin Sanabria1, María Teresa Alvarez Román2, Giancarlo Castaman3, Maissaa Janbain4,Tadashi Matsushita5,Karina Meijer6,Johannes Oldenburg7,Sabine Friedl8, Mark Reding9
1Bayer, Basel, Switzerland; 2Hospital Universitario La Paz, Madrid, Spain; 3Careggi University Hospital, Firenze, Italy; 4Tulane School of Medicine, New Orleans, USA; 5Nagoya University Hospital, Nagoya, Japan; 6The University Medical Center Groningen, Groningen, The Netherlands; 7University Clinic Bonn, Bonn, Germany; 8Bayer, Berlin, Germany; 9University of Minnesota Medical Center, Minneapolis, US
Disclosure of conflict of interest
Emilio Musi, Bayer employee.
Martin Sanabria, Bayer employee.
María Teresa Alvarez Román, Receipt of grants/research support: Shire/Takeda. Company sponsored speaker’s bureau: Amgen, Bayer, CSL Behring, Novartis, Novo Nordisk, Roche, Sobi, Shire/Takeda.
Giancarlo Castaman, Grants/research support: CSL Behring, Pfizer,Sobi. Honoraria or consultation fees: Roche. Company sponsored speaker’s bureau: Roche. Company sponsored speaker’s bureau: Bayer, CSL Behring, Kedrion – Shire, Novo Nordisk, Sobi, uniQure.
Maissaa Janbain, Grants/research support: HTRS-MRA (Bioverativ Sanofi). Honoraria or consultation fees: Bayer, CSL Behring, Genentech, Shire. Company sponsored speaker’s bureau: Shire (Vonvendi).
Tadashi Matsushita, Grants/research support: Bioverativ Sanofi, KM biologics. Honoraria or consultation fees: Bayer, Bioverativ Sanofi, Chugai, CSL Behring, Novo Nordisk, Pfizer, Shire.
Karina Meijer, Grants/research support: Bayer, Pfizer, Sanquin. Honoraria or consultation fees: uniQure.
Johannes Oldenburg, Honoraria or consultation fees: Bayer, Ceva Pharmaceuticals, Pfizer. Company sponsored speaker’s bureau: Bayer.
Sabine Friedl, Bayer employee and shareholder.
Mark Reding, Grants/research support: Bayer, BioMarin. Receipt of honoraria or consultation fees: Bayer, Bioverativ Sanofi, CSL Behring, Novo Nordisk Shire. Participation in a company sponsored speaker’s bureau: Bayer, Bioverativ Sanofi, Novo Nordisk, Shire.
Acknowledgements
Medical writing assistance was provided by Sudler Medical Communications and was fully funded by Bayer.
Background
- BAY 94-9027 (damoctocog alfa pegol), a site-specifically PEGylated B‑domain deleted recombinant factor VIII with an extended half-life, is approved for use in previously treated patients (PTPs) aged ≥12 years with haemophilia A in Canada, Japan, the EU and the US
- The efficacy and safety of BAY 94-9027 was demonstrated in 2 phase II/III clinical studies in PTPs with severe hemophilia A,1 however, real-world data are still being gathered
- The HEM‑POWR study will assess the effectiveness and long-term safety of BAY 94‑9027 in the real-world clinical setting2
- Patients will also be given the opportunity to participate in LIFE-ACTIVE, a sub-study analysing the relationship between the patients’ regular daily activity and the efficacy parameters collected during HEM-POWR
Aim
To describe the LIFE-ACTIVE rationale and design
Methods: HEM-POWR
- HEM-POWR (NCT03932201) is a multinational, multicentre, non-interventional, open-label, prospective, phase IV, cohort study
- It aims to enrol ≥200 PTPs with haemophilia A, receiving BAY 94-9027 (on-demand, prophylaxis, or intermittent prophylaxis [as per local label])
- Key exclusion criteria are presence or history of FVIII inhibitor (≥0.6 Bethesda units), diagnosis of any bleeding or coagulation disorder other than haemophilia A, or treatment with immune tolerance induction at enrolment
- The primary objective of HEM-POWR is to assess the effectiveness of prophylaxis with BAY 94-9027 in the real-world setting through the collection of total bleeding events and analysis of annualised bleeding rate
- Secondary objectives include long-term safety, joint health, location and number of target joints, haemostasis during surgery and patient-reported outcomes
LIFE-ACTIVE
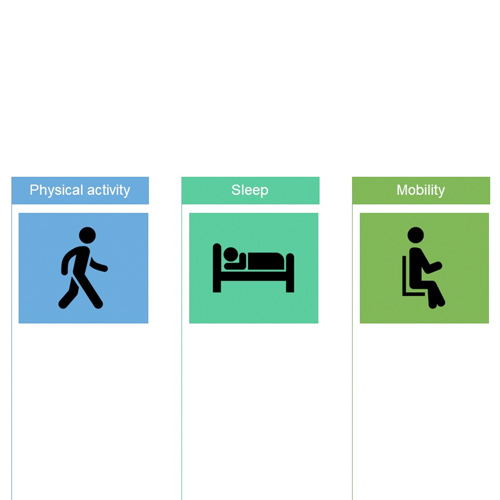
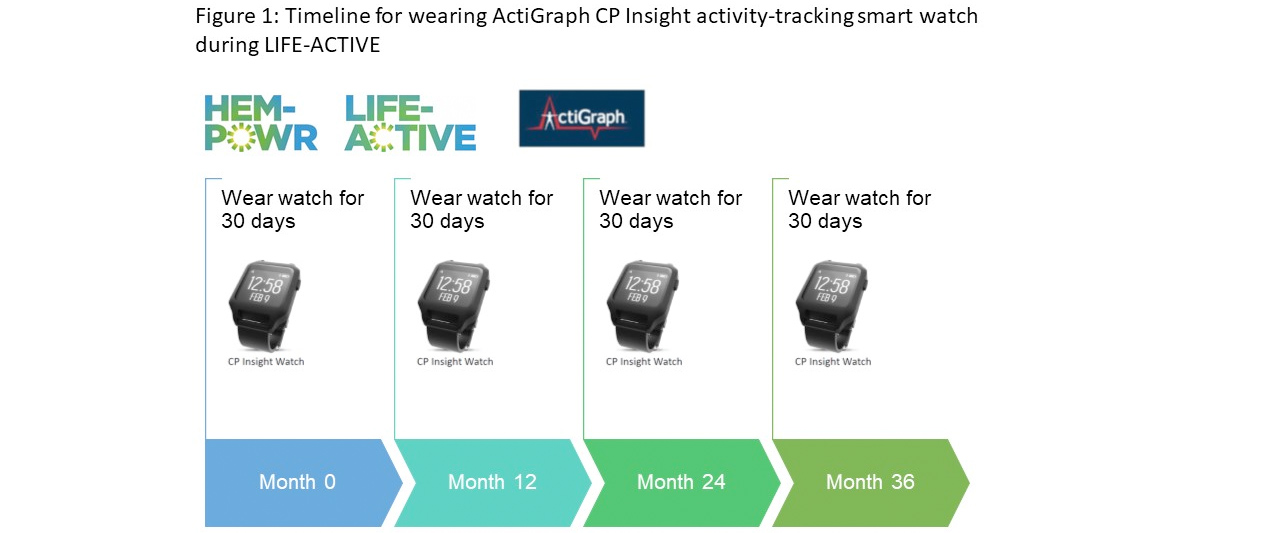
- Patients participating in an optional LIFE-ACTIVE sub-study will be asked to wear an ActiGraph CP Insight activity-tracking smart watch, continually for four 30-day periods, at their initial visit and then at Months 12, 24 and 36 (Figure 1)
- Measurements recorded will include physical activity intensity and duration, general mobility and sleep quality and duration
- All data will be transferred to the secure, cloud-based CentrePoint system and patients will not be aware of the values measured by the device
Results
- It is expected that this innovative data collection approach will provide robust data in near real time
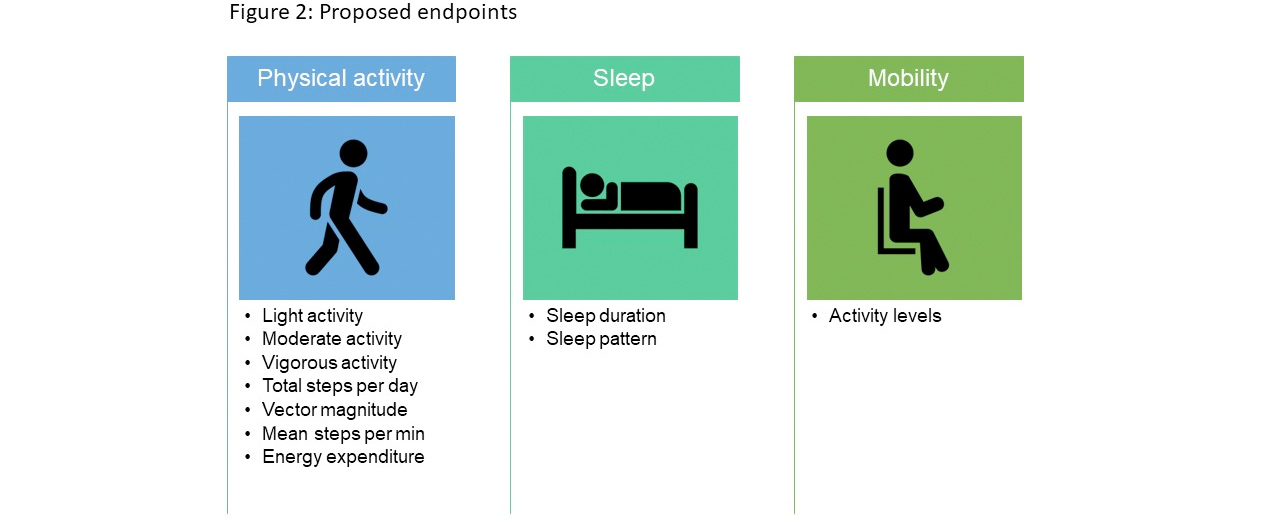
- Proposed endpoints include physical activity, total steps (per minute/per day) and energy expenditure (Figure 2)
- This sub-study will investigate the relationship between daily regular activity, as measured by ActiGraph, and effectiveness parameters collected from the HEM-POWR study (including quality of life and bleeding rates)
- It is expected that positive changes, such as an increase in sleep length and increases in physical activity would correlate with an increased quality of life and improvement in clinical parameters
Conclusions
- ActiGraph will provide thorough, robust activity measurements, helping to accurately assess quality of life
- LIFE-ACTIVE will provide insights into patterns of physical activity and sleep, and their relationship with clinical outcomes
- This sub-study will also demonstrate how technology such as smart watches can improve data measurements and may positively influence patient compliance
References
1.Reding, et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost 2017;15:411–9.
2.ClinicalTrials.gov identifier: NCT03932201. Available at: https://clinicaltrials.gov/ct2/show/NCT03932201. Accessed July 2019.