Andreas Baumann
Bayer AG
13342 Berlin, Germany
+49 30 468-17450
andreas.baumann@bayer.com
Disclosure of conflict of interest
The author is an employee of Bayer.
Acknowledgements
Medical writing support was provided by Shakirah Chowdhury of Darwin Healthcare.
Background
- The addition of polyethylene glycol (PEG) to molecules (PEGylation) is a valuable technique to extend the half-life of various biologics
- PEGylation has been shown to increase the hydrodynamic radius of a protein, thereby improving drug stability and reducing receptor interaction1
- BAY 94-9027 is an extended half-life, B-domain deleted recombinant factor VIII (FVIII) that is site-specifically PEGylated with a branched 60 kDa (2×30 kDa) PEG
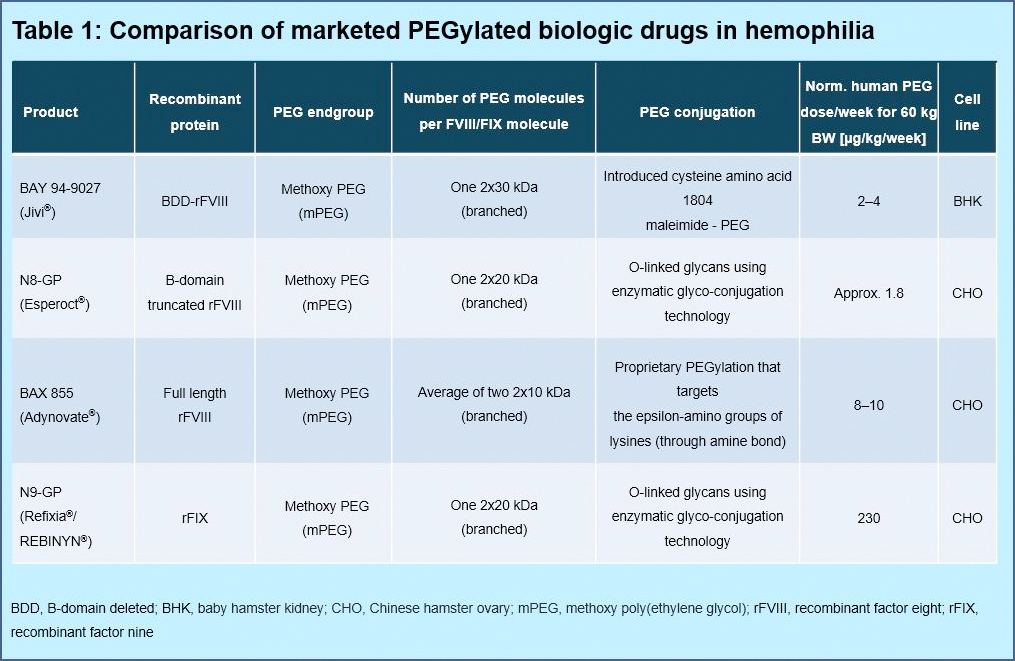
- PEGylated products have been used for more than 20 years with more than 12 products approved in Europe and the US in different indications (Table 1). No safety concerns related to long-term PEG exposure have been identified in humans
Aims
To discuss the safety of PEG, particularly in the context of BAY 94-9027, and address potential concerns surrounding the possibility of PEG accumulation in the body
Methods
- There are four main factors to consider when evaluating the long-term safety profile of PEGylated biologics in hemophilia treatment: regulatory aspects, non-clinical safety, pharmacokinetics of PEG, and clinical studies
- Data from these four areas in relation to long-term PEG safety are presented and discussed
Results
Regulatory requirements
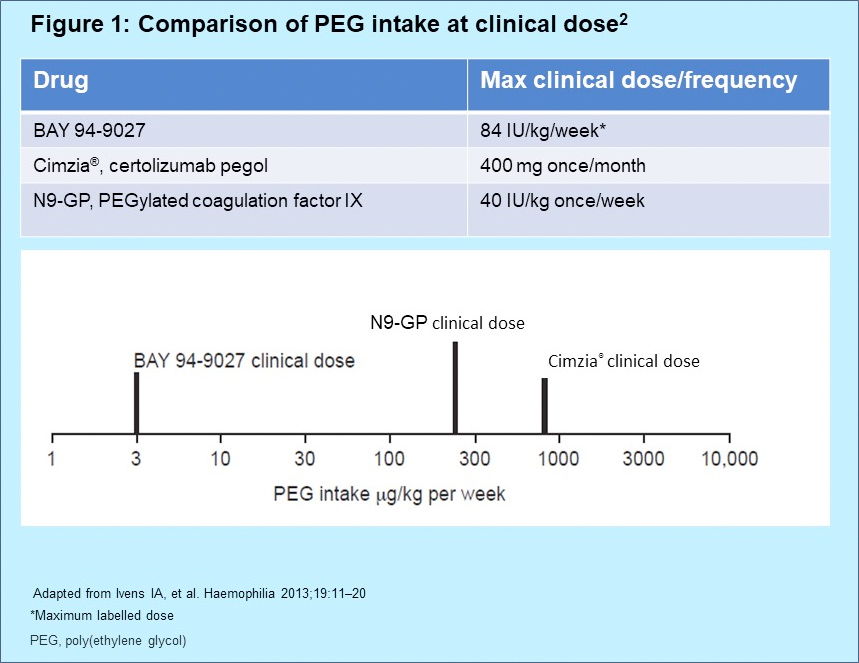
- A maximum acceptable administrable monthly dose of PEG (e.g. as part of a PEGylated molecule) of 0.4 μmol/kg/month has been defined for the pediatric population by the Committee for Medicinal Products for Human Use (CHMP) Safety Working Party’s paper3
- Cellular vacuolation within renal tubule and/or choroid plexus ependymal cells has been observed in repeat-dose toxicity studies with proteins PEGylated with molecules ≥ 40 kD when the administered dose gave rise to at least a monthly PEG exposure of 0.4 μmol/kg/month,4 which would equate to 24,000 µg/kg/month of PEG-60 in BAY 94-9027
- Regulatory Authority’s recommendation: Before any clinical study of PEGylated products for treatment periods longer than four weeks is conducted, it should be addressed whether:
- Ependymal cell vacuolation has been observed in non-clinical studies and
- The PEGylated drug may undergo active transport across the blood-CSF barrier3
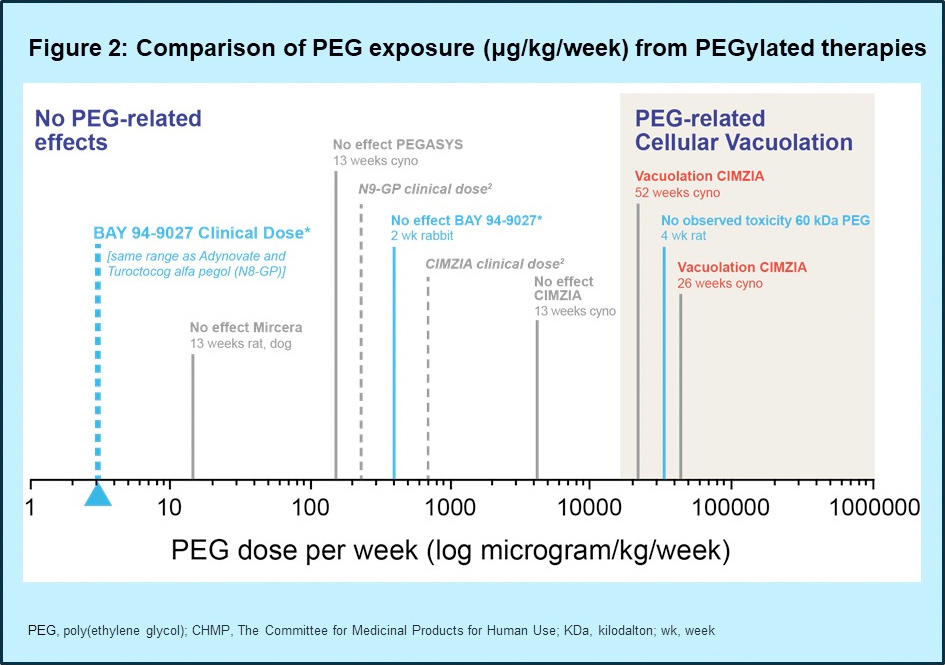
- Based on theoretical calculations, PEG intake with BAY 94-9027 is 750× lower than the EMA defined threshold for cellular changes
Non-clinical safety4
- No vacuolation was detected by light microscopy in any organ in immunodeficient rats after chronic (6 months) administration of BAY 94-9027, administering of doses up to 30× higher than that of the human dose
- No BAY 94-9027-related changes were recorded during in-life or recovery phase in all doses, including the highest dose of 1200 IU/kg twice weekly. No PEG was detected in choroid plexus or other areas of the brain, cerebrospinal fluid or in spleen or kidneys
- No PEG-related vacuolation is expected at clinical doses of BAY 94-9027 (Figure 2)
Pharmacokinetics of PEG
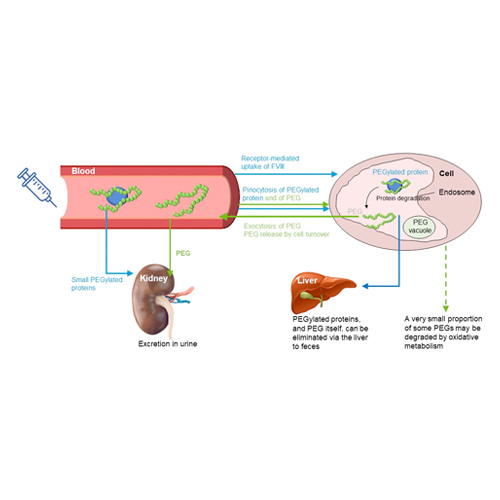
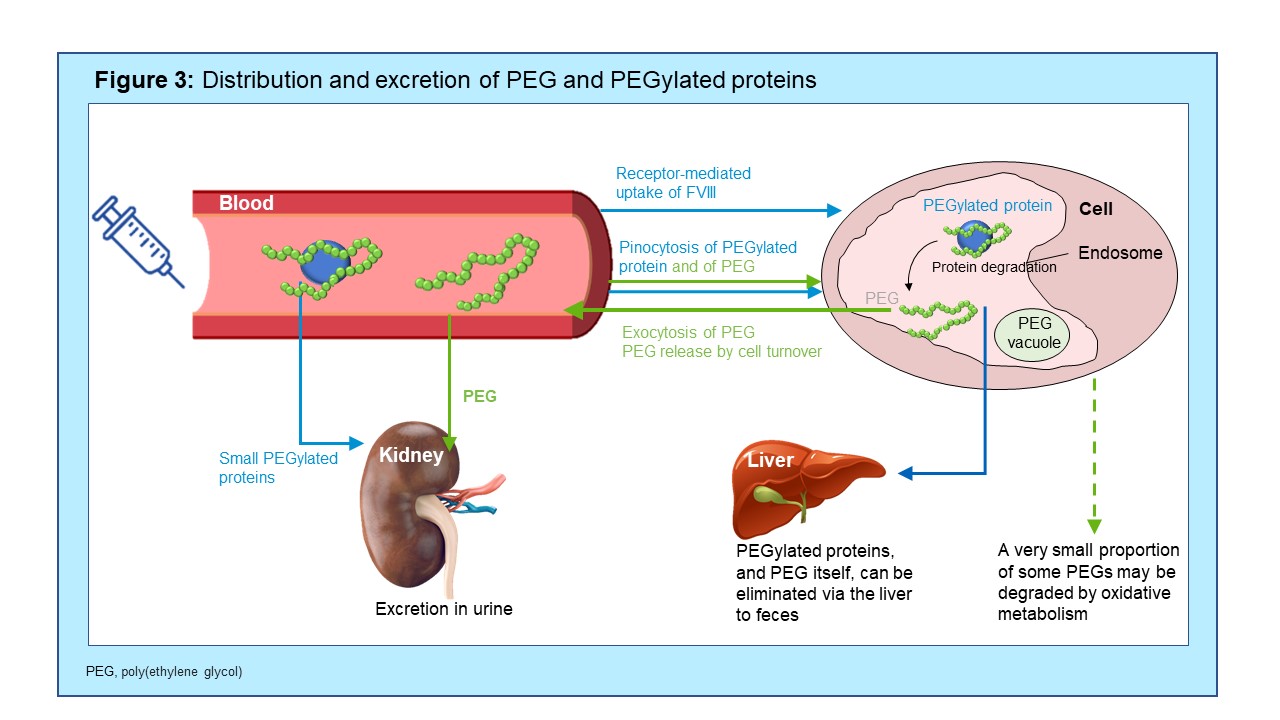
- Since excretion processes for large PEGs including PEG-60 have been demonstrated (figure 3), once a steady state is reached, there are no further increases in blood and/or tissue concentrations5
- Steady-state level is dependent on PEG load (dose) and is important for the prediction of long-term safety
- Based on pre-clinical data, PEG-60 plasma steady state levels after human dosing of BAY 94-9027 can be well predicted
Clinical studies
- There is a clear relationship between PEG dose and plasma steady-state levels for different PEGylated products
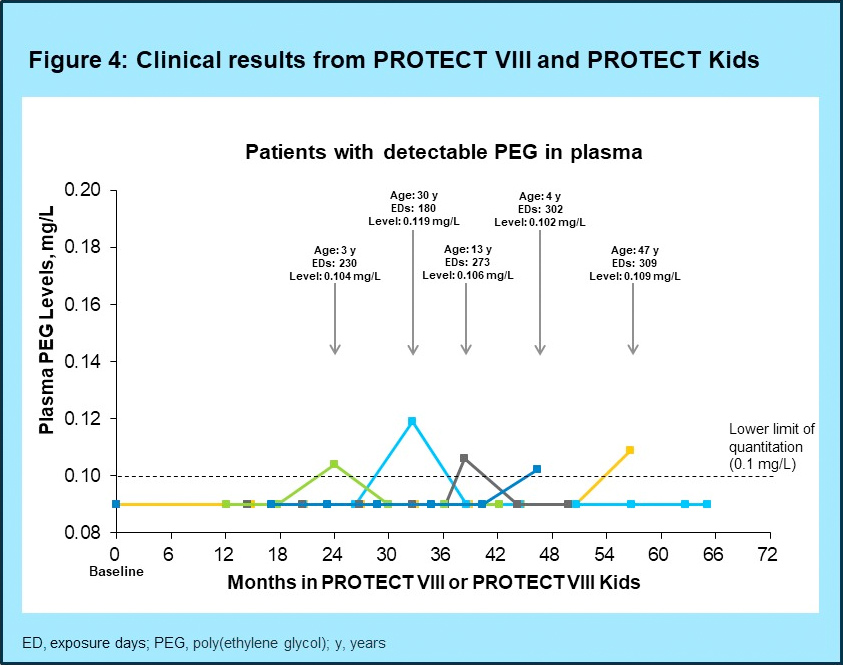
- Plasma PEG levels predicted from pre-clinical studies were confirmed by clinical studies of long-term exposure to BAY 94-9027; PEG levels were undetectable at all visits for 173/179 patients. Transient positive results were only detected in 6 patients and were just above the LLOQ (study duration 0.8‒5.4 years (Figure 4)
- Combined with the demonstrated excretion mechanism of PEGs up to 60 kDa, the very low intake of PEG is not expected to have any long-term safety consequences, as confirmed by clinical data on the use of BAY 94-9027 for more than five years in the PROTECT VIII and PROTECT VIII Kids trials5
Conclusions
- Predictions for the safety of long-term prophylactic use of PEGylated biologics in humans must consider regulatory requirements, nonclinical safety, pharmacokinetics, clinical experience
- No PEG-related vacuolation is anticipated at the clinical dose of BAY 94-9027, nor was it observed in any toxicology study, including chronic administration
- PEG levels were below, or at the lower limit of detection (in only a few cases), after long-term treatment with BAY 94-9027 for more than 5 years of clinical experience. No renal or neurological AE were associated with long-term use of BAY 94-9027
References
- Lawrence PB and Price JL. Curr Opin Chem Biol. 2016; 34;88–94.
- Ivens IA, et al. Haemophilia. 2013; 19:11–20.
- European Medicines Agency. CHMP Safety Working Party’s response to the PDCO regarding the use of PEGylated drug products in the paediatric population. 2012 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/11/WC500135123.pdf Accessed July 2019.
- Ivens IA, et al. Toxicol Pathol, 2019;1–13.
- Baumann A, et al. Eur J Pharm Sci, 2019;130:11–20.