| Arch of Constantine | Vittoria Bridge | |
| 11:00 | Bleeding phenotype and target joints predict patients with zero bleeds given once-weekly rophylaxis with BAY94-9027 E. Musi (Switzerland) |
Evaluation of a fully–automated
chemiluminescent immunoassay for the rapid quantification of ADAMTS13
activity and the detection of ADAMTS13 inhibitors M. Mirabet (Spain) |
| 11:15 | Comparing the one-stage and
chromogenic assay: actor VIII activity assay discrepancy at baseline
does not eflect assay discrepancy after desmopressin in non-severe
hemophilia A patients. L. Schutte (the Netherlands) |
Immunoglobulin G subclass
distribution of anti-ADAMTS13 antibodies and its association with
HLA-DR-DQ haplotypes and clinical course in acquired thrombotic
thrombocytopenic purpura G. Sinkovits (Hungary) |
| 13:00 | BAY 1093884 target the Kunitz Domains 1 and 2 of TFPI and Blocks Its Function P. Mathew (USA) |
Congenital (hereditary) thrombotic
thrombocytopenic purpura (cTTP [hTTP], Upshaw–Schulman Syndrome):
Patient experience, conceptual framework, and patient-reported outcome
(PRO) instrument development B. Ewenstein (USA) |
| 13:15 | Nonacog beta pegol in adult andpaediatric patients: pooled data from the paradigm™ clinical programme M. Lapecorella (Italy) |
Unraveling anti-spacer immunoprofiles of acquired TTP patients using antiidiotypic antibodies A. S. Schelpe (Belgium) |
| 13:30 | Preliminary enrolment data from the U.S. post-marketing safety (PMS) study of rpFVIII in patients with acquired hemophilia R. Crea (Austria) |
Prevention of relapses in patients affected by acquired TTP undergoing elective surgery B. Ferrari (Italy) |
| 14:00 | Nonacog beta pegol for the
prophylactic treatment of children with hemophilia B: interim results
from the Paradigm™5 clinical trial M. Lapecorella (Italy) |
Possibile repeat time |
| 14:15 | Possibile repeat time | Possibile repeat time |
| 16:30 | Clustered F8 missense mutations cause
hemophilia A phenotypic heterogeneity by combination of altered
splicing, protein secretion and activity M. Pinotti (Italy) |
Diagnosis of Upshaw-Schulman syndrome in adulthood B. Ferrari (Italy) |
| 16:45 | Von Willebrand factor levels in patients with hemophilia A. M. Milos (Croatia) |
Modeling-guided identification of structural determinants contributing to conformational changes within ADAMTS13 B. Ercig (the Netherlands) |
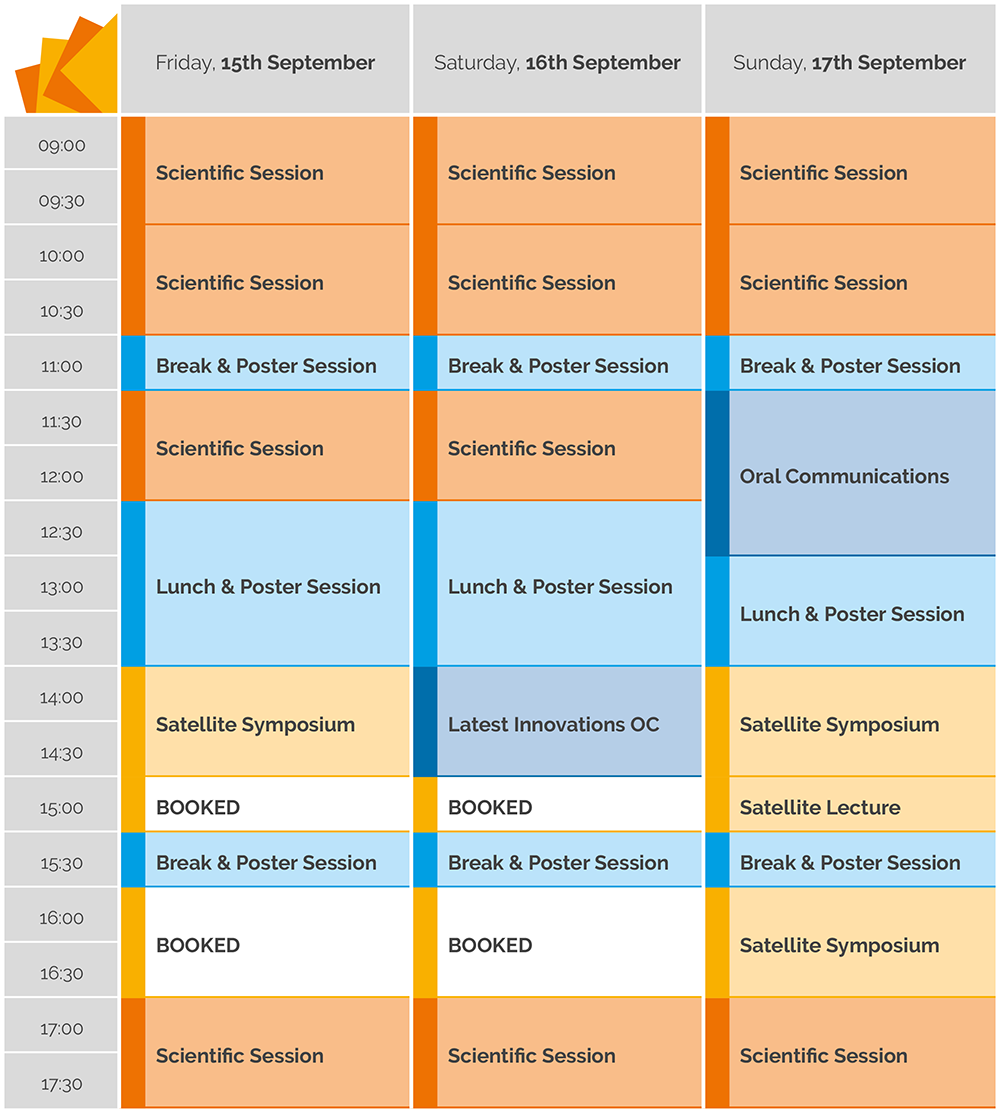
ePOSTER PROGRAMME
FRIDAY, 15th September
SATURDAY, 16th September
| Arch of Constantine | Vittoria Bridge | |
| 11:00 | Whole exome sequencing as a first tier test in diagnosing primary bleeding disorders: WES First Y. Smit (the Netherlands) |
Quantitative ELISA assay for in vivo
proteolysis of von Willebrand factor and bleeding: A pilot study in type
2A(IIA) von Willebrand disease S. Susen (France) |
| 11:15 | The rare coagulation factor deficiencies: Single centre experience F. D. Koseoglu (Turkey) |
Development of a gene therapy strategy for von Willebrand disease based on dual adeno-associated virus vectors E. Barbon (France) |
| 13:00 | Possible repeat time | Efficacy, safety, and presurgical
pharmacokinetics of recombinant VWF (rVWF) in patients with severe VWD
who are undergoing elective surgical procedures F. Peyvandi (Italy) |
| 13:15 | Effectiveness and safety of rFVIIa in paediatric Glanzmann Thrombasthenia patients: data from the international Glanzmann Thrombasthenia Registry A. Roveda (Italy) |
Laboratory diagnosis of von Willebrand disease type 2A versus LVAD-induced acquired von Willebrand syndrome S. Deconinck (Belgium) |
| 13:30 | Rare bleeding disorders in the Netherlands J. Saes (the Netherlands) |
Differential diagnosis between type 2A and 2B VWD in a 2-years old female child with a de novo novel mutation M. T. Pagliari (Italy) |
| 14:00 | Simultaneous measurement of thrombin and plasmin generation in patients with Factor XI deficiency J. Saes (the Netherlands) |
Platelet function analyzer
measurement of closure time as a biomarker for activity of high and
ultralarge multimers of recombinant von Willebrand factor (rVWF) P. Turecek (Austria) |
| 14:15 | Possibile repeat time | Possibile repeat time |
| 16:00 | Acquired factor V inhibition due to Bactrim therapy – A previously unreported cause of a rare coagulopathy R. Gately (Australia) |
Usefulness of von Willebrand factor
propeptide in the differential diagnosis between von Willebrand disease
and acquired von Willebrand syndrome F. Stufano (Italy) |
| 16:15 | Hepatitis C viral infection in patients with hemophilia; An experience of Ege Adult Haemophilia Center F. D. Koseoglu (Turkey) |
Recombinant human von Willebrand
factor has a unique pattern of ultra large multimers: results from
physico, biochemical and in vivo studies P. Turececk (Austria) |
SUNDAY, 17th September
| Arch of Constantine | Vittoria Bridge | |
| 11:00 | Development of inhibitors in PUPs:Different scenario, same outcome S. Trakymiene (Lithuania) |
Soluble glycoprotein VI (sGPVI)
measurement is a useful biomarker of platelet activation in
heparin-induced thrombocytopenia (HIT) and correlates with thrombotic
events. C. W. Tan (Australia) |
| 11:15 | A novel macrophage-mediated pathway regulates enhanced clearance of hyposialylated von Willebrand factor in vivo J. O’Sullivan (Ireland) |
Child-onset thrombotic thrombocytopenic purpura: the French Reference Center for Thrombotic MicroAngiopathies experience B. Joly (France) |
| 13:00 | Stabilin-2 deficiency increases procoagulant activity and deep vein thrombosis in mice A. Michels (Canada) |
Life-threatening pregnancy-associated atypical haemolytic uraemic syndrome and its response to eculizumab R. Gately (Australia) |
| 13:15 | Enhanced local disorder in a clinically elusive von Willebrand factor provokes high-affinity platelet clumping M. Auton (USA) |
Possible repeat time |
| 13:30 | A cell-based assay to quantify von Willebrand factor mutant binding to integrin αIIbβ3 M. A. Brehm (Germany) |
Possible repeat time |
| 14:00 | Variability in blood outgrowth endothelial cell characteristics and related von Willebrand factor parameters A. De Jong (the Netherlands) |
Possible repeat time |
| 16:30 | Possible repeat time | Possible repeat time |
| 16:45 | Possible repeat time | Possible repeat time |