Marija Milos1, Désirée Coen Herak1, Jovan P. Antovic2, Nida Mahmoud Hourani Soutari2, Josipa Pavic3, Silva Zupancic-Salek4, Renata Zadro1,5
1Department of Laboratory Diagnostics, University Hospital Centre Zagreb, Zagreb, Croatia; 2Department of Coagulation Research, Institute for Molecular Medicine and Surgery, Karolinska Institutet & Department of Clinical Chemistry, Karolinska University Hospital, Stockholm, Sweden; 3Department of Medical Biochemistry and Hematology Laboratory, General County Hospital Livno, Livno, Bosnia and Herzegovina; 4Department of Medicine, University Hospital Centre Zagreb, Zagreb, Croatia; 5University of Zagreb, Faculty of Pharmacy and Biochemistry, Zagreb, Croatia
BACKGROUND / AIMS
Traditionally used laboratory methods, i.e. one-stage or chromogenic assays for FVIII activity determination do not always and accurately reflect bleeding severity in haemophilia A (HA) patients. As global haemostasis assays provide overall haemostatic status estimation, we investigated the ability of three global assays for identifying bleeding phenotype both in severe and non-severe haemophilia A patients, as well as their usefulness in laboratory management of haemophilia A patients with discrepant bleeding phenotype.
MATERIALS AND METHODS
♥ PATIENTS & CONTROLS
- 62 haemophilia A patients
- 30 severe haemophilia A patients
- 32 non-severe haemophilia A patients
- 27 healthy male controls
♥ METHODS
- Global haemostasis assays
- Overall haemostasis potential (OHP)
- aPTT-clot waveform analysis (DELTA)
- endogenous thrombin potential (ETP)
♥ SCORING METHOD
classification of haemophilia A patients regarding bleeding phenotype included three clinical parameters:
- age at first joint bleed
- number of target joints
- number of joint/muscle bleeds per year
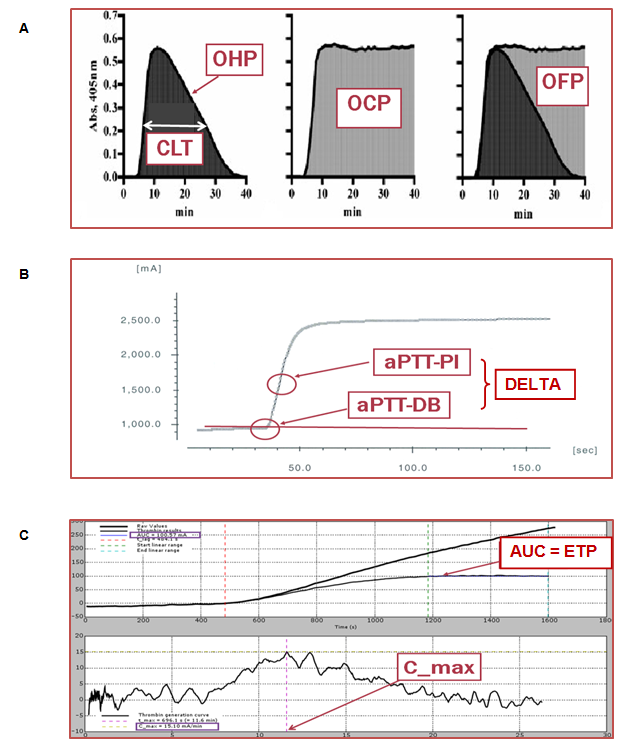
FIGURE 1 Global haemostasis assays used in this study: overall haemostasis potential – OHP (A),
aPTT-clot waveform analysis – DELTA (B) and endogenous thrombin potential – ETP (C)
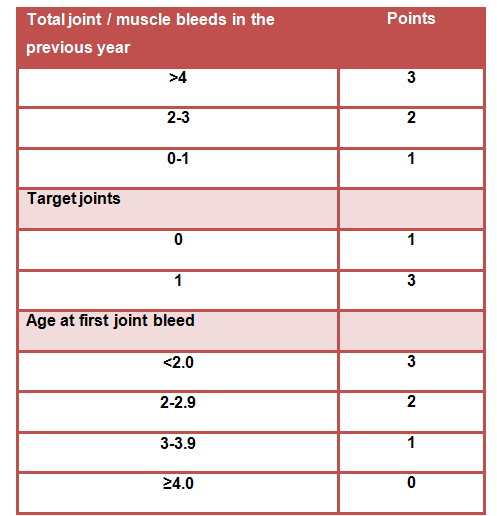
TABLE 1 Applied scoring method for bleeding phenotype determination
Scoring: ≥5 points suggest a severe bleeding phenotype
≤4 points suggest a mild bleeding phenotype
RESULTS
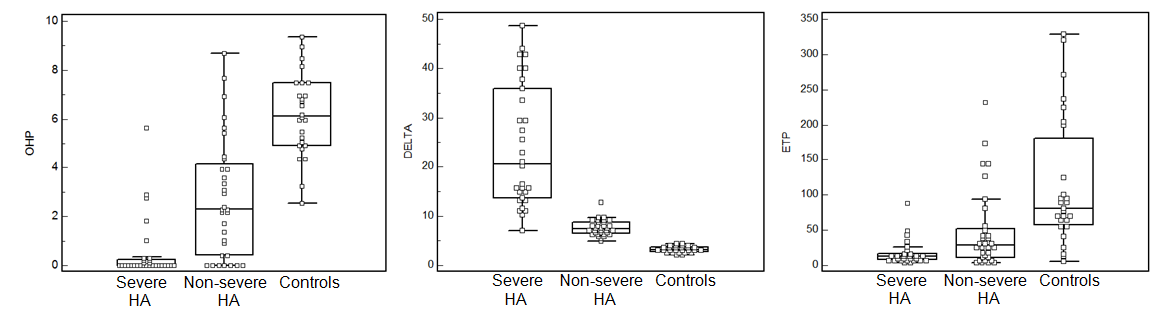
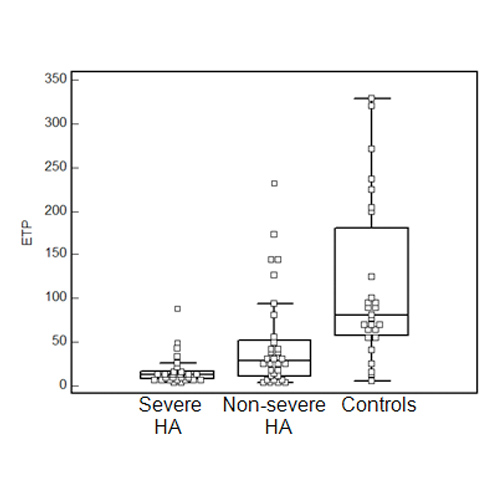
FIGURE 2. Box & Whisker plots of overall haemostasis potential (OHP), aPTT-waveform analysis (DELTA) and endogenous thrombin potential (ETP) in severe and non-severe hemophilia A (HA) patients and controls.
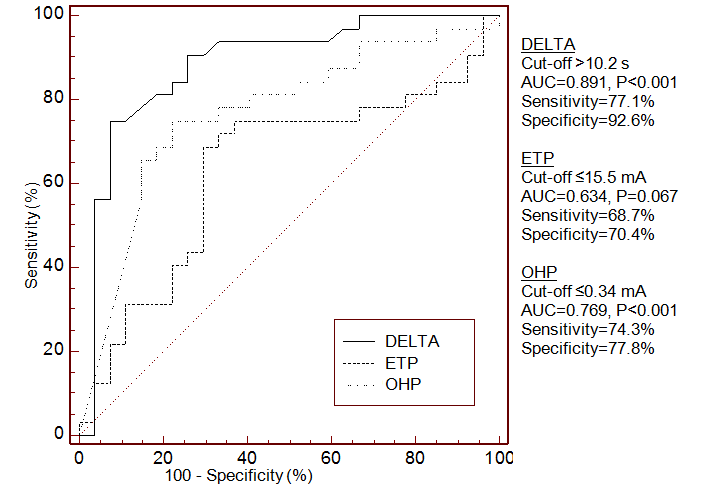
FIGURE 3 Receiver Operating Characteristic curves determining the ability of aPTT-waveform analysis (DELTA), endogenous thrombin potential (ETP) and overall haemostasis potential (OHP) to distinguish between patients with severe and mild bleeding phenotype
AUC – area under the curve
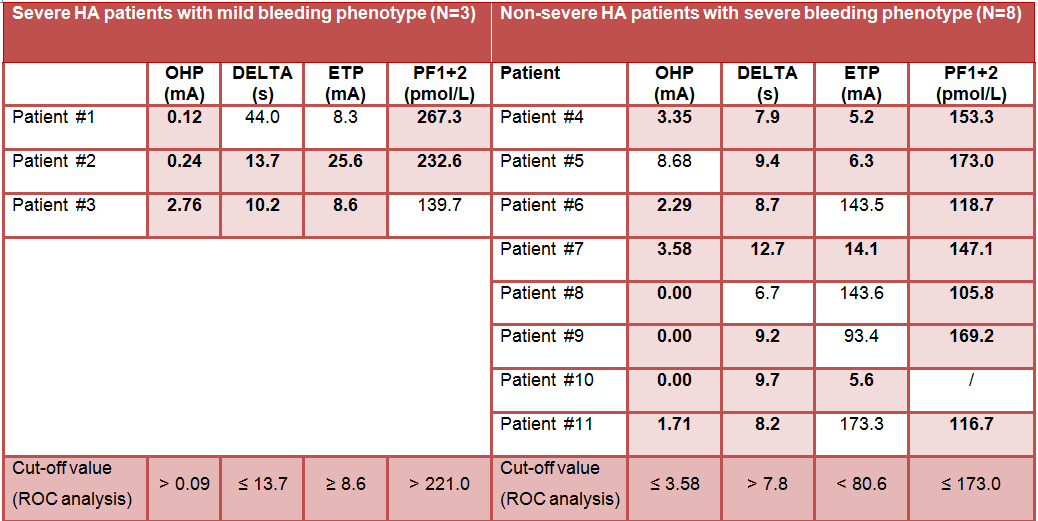
TABLE 2 Results of global assay parameters and prothrombin fragment 1+2 for discrepant patients (N=11), i.e. severe haemophilia A patients with mild bleeding phenotype and non-severe haemophilia A patients with severe bleeding phenotype
CONCLUSIONS
- the present study confirmed the significant correlation of OHP, DELTA and ETP with FVIII activity
- OHP, DELTA and ETP enabled clear discrimination between severe and non-severe HA patients and controls, although some overlaps of results were noticed
- ROC analysis for OHP, DELTA and ETP yielded remarkable sensitivities and specificities in discriminating between patients with severe and mild bleeding phenotype
- OHP, DELTA and ETP enabled detection of 11/11 patients with discrepant bleeding phenotype. The best results were achieved by combining OHP and aPTT-CWA.
⇒ GLOBAL ASSAYS ARE SUPERIOR BLEEDING PHENOTYPE DETERMINANTS IN A MAJORITY OF INDIVIDUAL HA PATIENTS, COMPARED TO FVIII ALONE.
⇒ GLOBAL ASSAYS SHOULD BE INCLUDED IN THE DIAGNOSTIC ALGORITHM AT LEAST IN PATIENTS WITH DISCREPANT RESULTS BETWEEN FVIII ACTIVITY AND CALCULATED BLEEDING SCORE.
⇒ IMPLEMENTATION OF GLOBAL ASSAYS ALLOWS EARLY RECOGNITION OF SEVERE HA PATIENTS WITH MILD BLEEDING PHENOTYPE THAT MAY NEED LOWER TREATMENT REGIMEN, AS WELL AS NON-SEVERE HA PATIENTS WITH SEVERE BLEEDING PHENOTYPE THAT ARE AT INCREASED BLEEDING RISK.
⇒ CONSIDERING THAT GLOBAL ASSAYS ARE STILL NOT AVAILABLE IN ALL COAGULATION LABORATORIES, WE SUGGEST THEIR IMPLEMENTATION IN CLINICAL PRACTICE AT LEAST IN LABORATORIES THAT ARE PART OF COMPREHENSIVE HAEMOPHILIA CENTERS.