Simone Merlin1 , Tamara Garcia-Leal2, Silvia Buzzi 1, MaríaJosé Sánchez2*, Antonia Follenzi1*
1Department of Health Sciences, University of Piemonte Orientale “A. Avogadro”, Novara, Italy; 2Centro Andaluz de Biología del Desarrollo (CABD), Consejo Superior de Investigaciones Científicas (CSIC), Junta de Andalucía, Pablo de Olavide University, Seville, Spain
*co-senior authors
BACKGROUND
Hemophilia A cell therapy approaches in paediatric individuals require suitable FVIII-producing cell populations presenting stable engraftment potential. We previously showed that adult-derived FVIII-producing cells, i.e. liver sinusoidal endothelial cells (LSEC) and hematopoietic stem cells (HSC), can be used for the treatment of adult hemophilia A (HA) mice. However, after transplantation in busulfan-conditioned newborn mice, adult LSEC/HSC cannot efficiently engraft, while murine fetal liver (FL) hemato/vascular cells from day 11–13 of gestation (E11-E13) strongly reconstitute the hematopoietic compartment and showed multiorgan endothelial reconstitution potential while secreting FVIII.
AIMS
To investigate the engraftment of FL cells in newborn HA mice as a new strategy to develop an experimental treatment for HA in neonates.
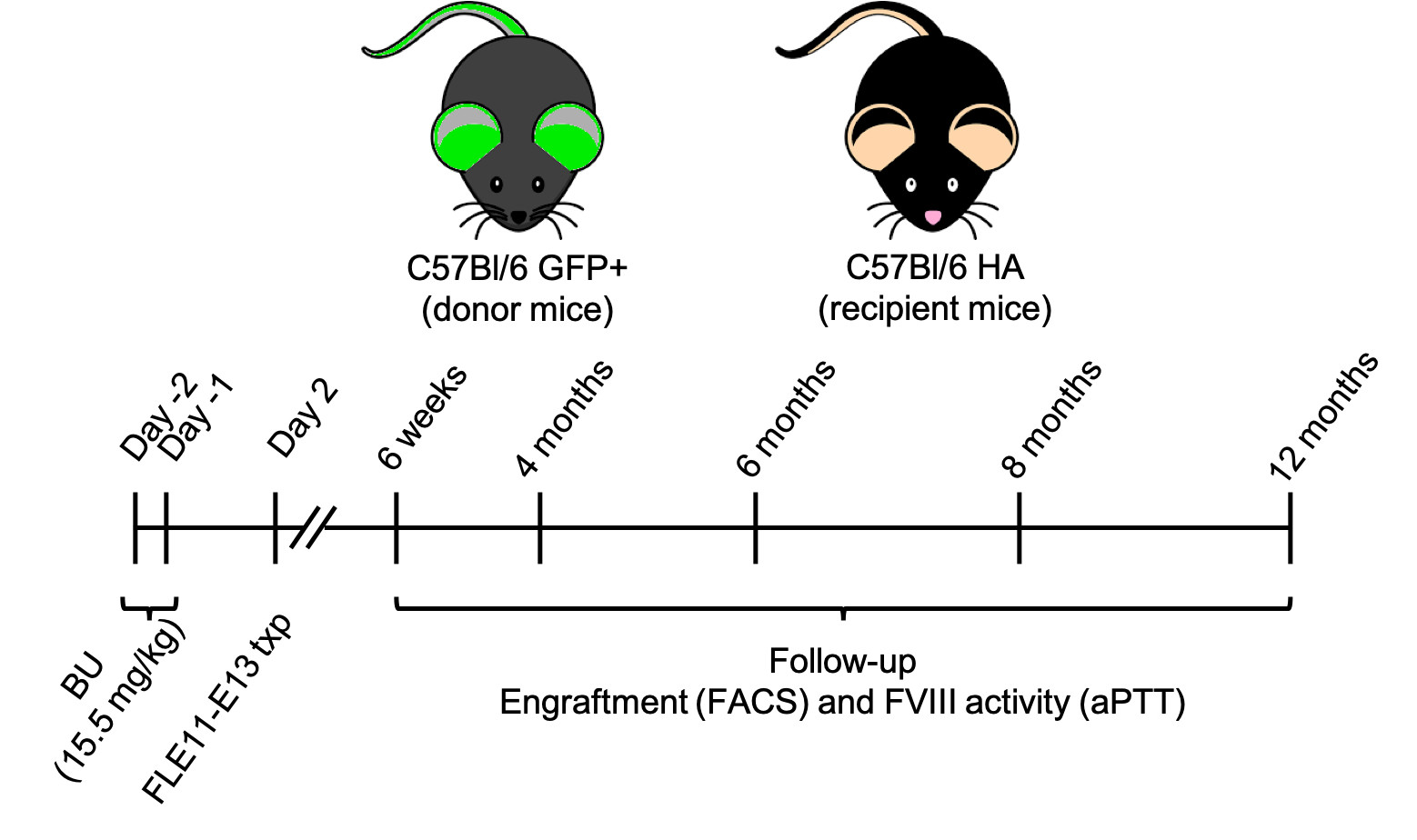
Figure 1. Schematic representation of the experimental procedure. HA mice received BU 2d and 1d before birth. 2d after birth HA mice were transplanted with FL E11-E13 cells from GFP+ mice.
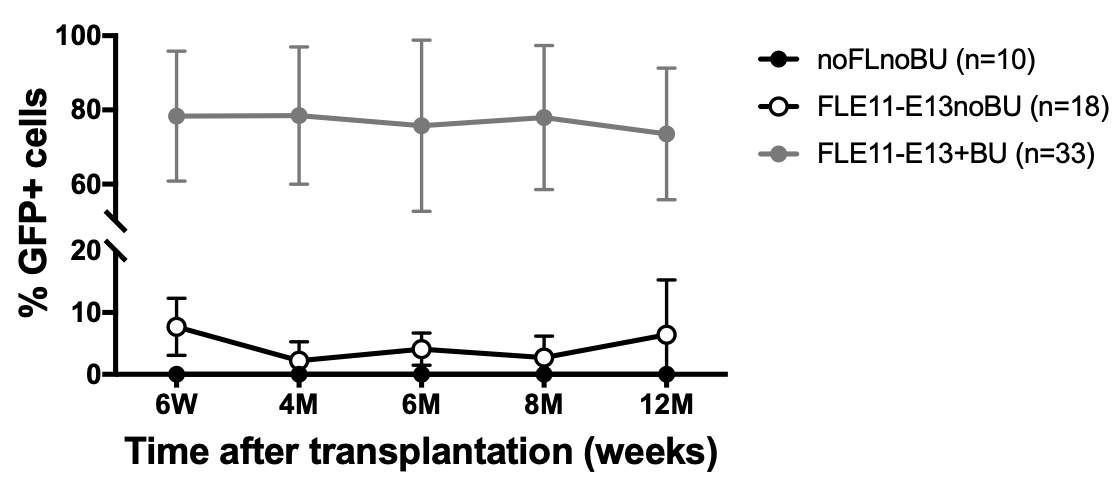
Figure 2. Engraftment of transplanted GFP+ cells was evaluated by flow cytometry on peripheral blood cells at different time points up to 1 year after transplantation. GFP+ cells were detectable in peripheral blood of all transplanted mice. Pre-treatment with BU drastically increased the engraftment of transplanted cells.
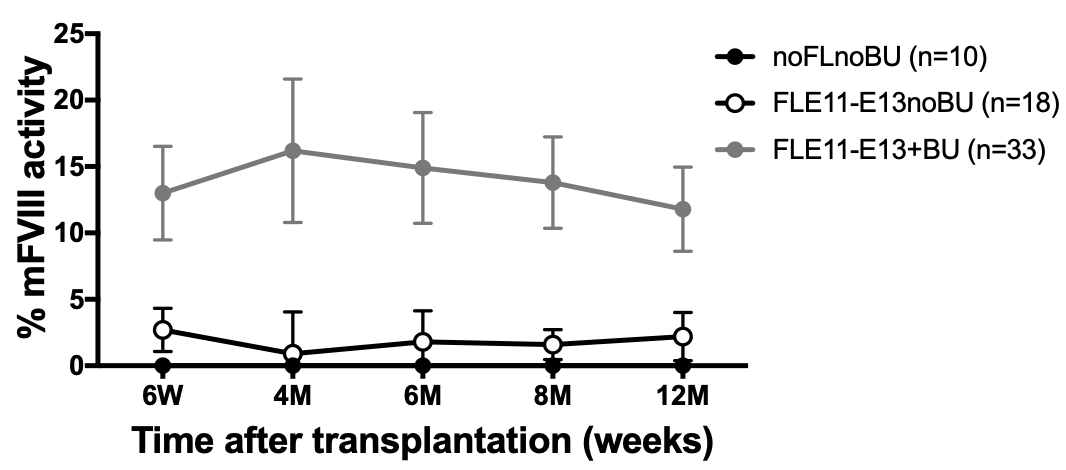
Figure 3. FVIII activity in plasma of transplanted mice was evaluated along with engraftment by aPTT assay. Murine FVIII activity was detectable up to 1 year in all transplanted mice and FVIII levels correlated with percentage of engraftment.
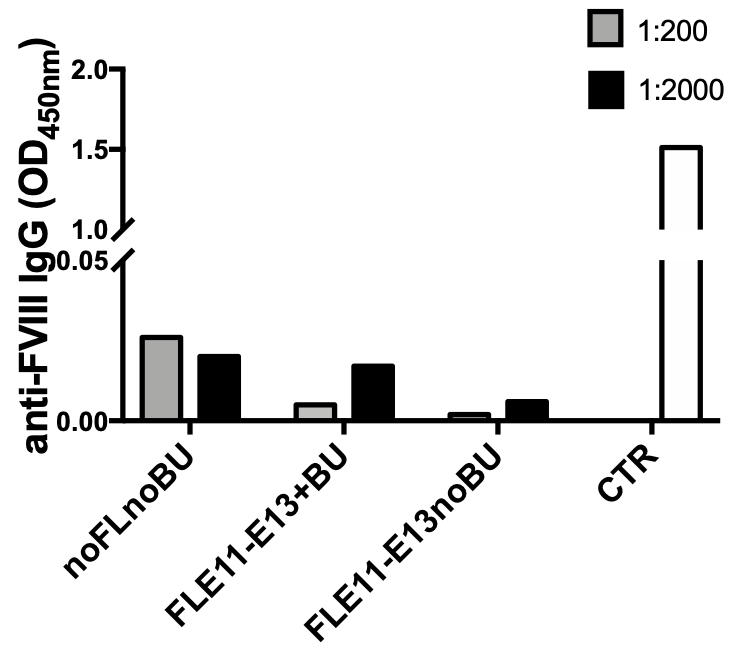
Figure 4. Presence of anti-FVIII antibodies was evaluated in plasma of treated mice by ELISA. No anti-FVIII antibodies were detected in plasma of treated mice from all groups. Results show analysis performed on plasma samples collected 10 months after transplantation. 1:200 and 1:2000 = plasma dilutions.
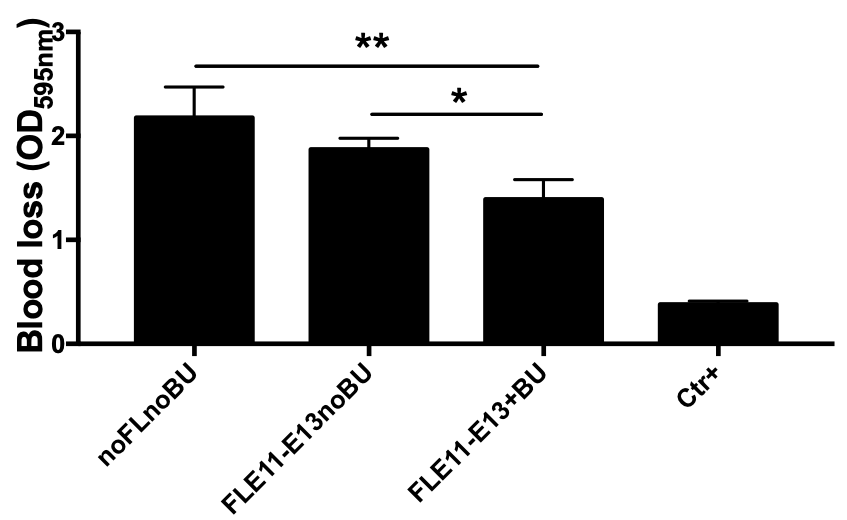
Figure 5. Bleeding assay performed 1 year after transplantation further confirmed the correction of the bleeding phenotype in mice transplanted with FL cells and the correction correlated with FVIII levels measured. A signifiative reduction in blood loss was observed in FLE11-E13+BU compared to noFLnoBU or FLE11-E13noBU groups, while a non significative reduction in blood loss was observed in FLE11-E13noBU mice compared to noFLnoBUgroup.
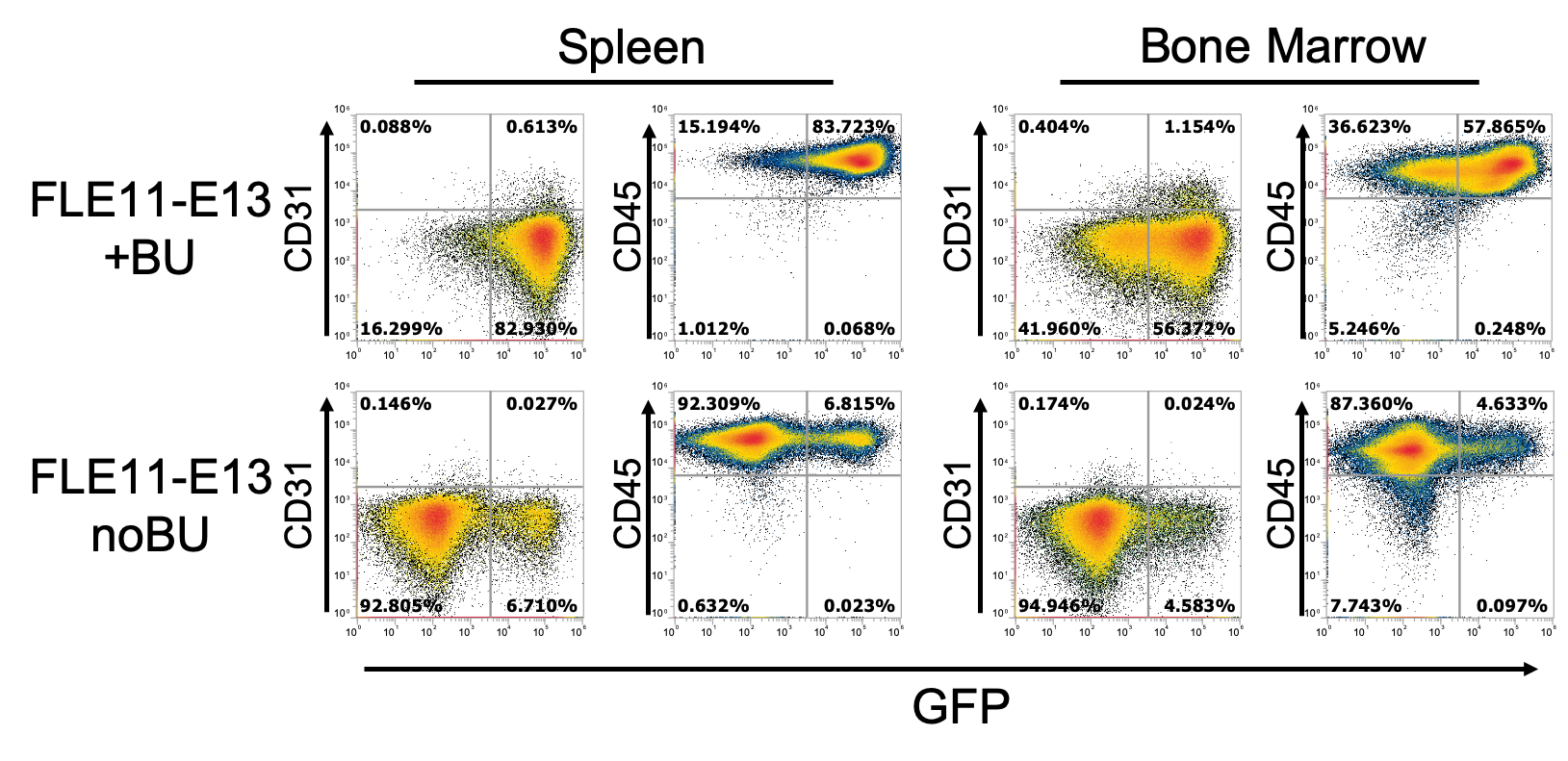
Figure 6. Flow cytometry analysis on spleen and bone marrow of transplanted mice showed the GFP+ cells were mainly of hematopoietic oring (CD45+) in both groups of transplanted mice FLE11-E13noBU and FLE11-E13.
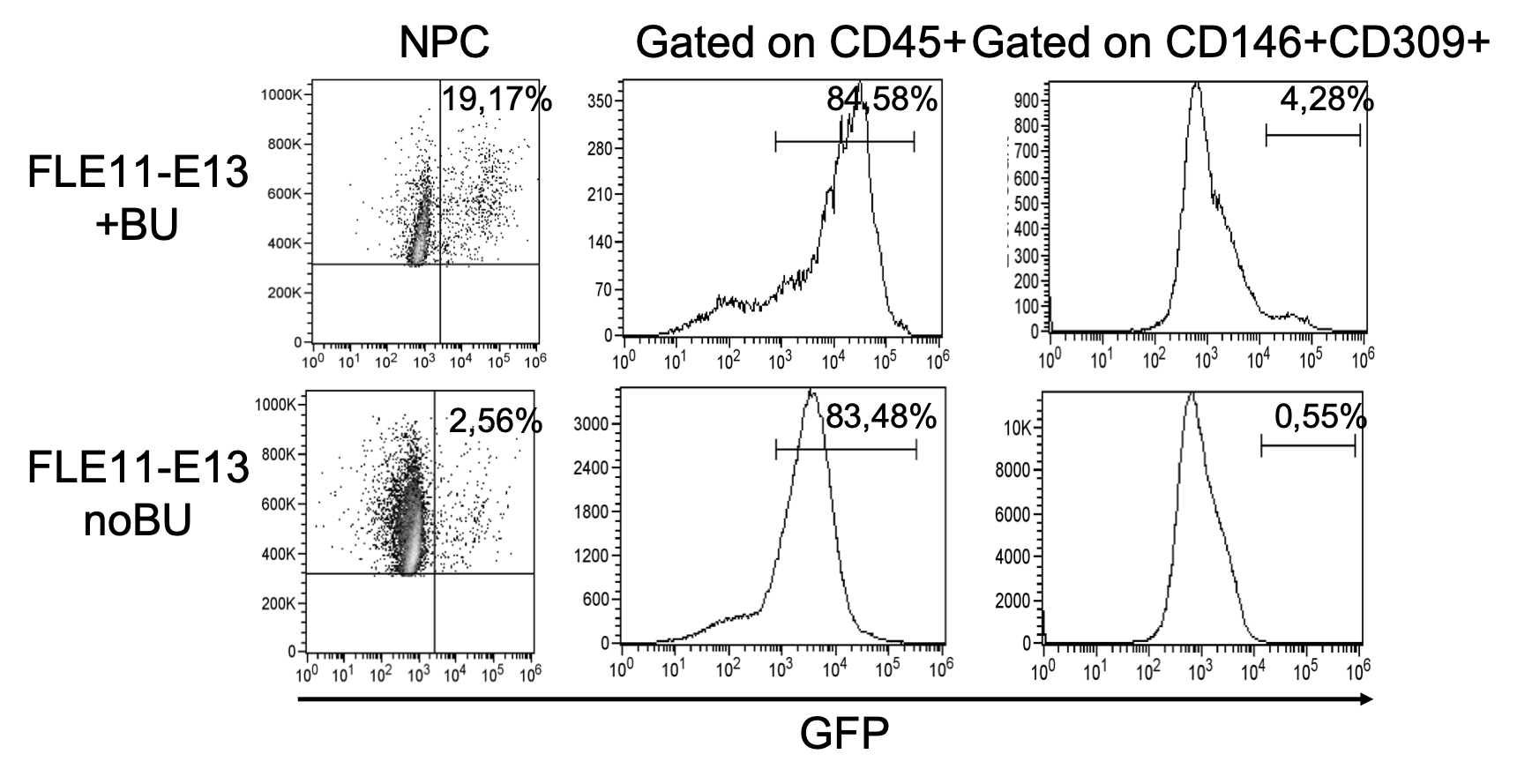
Figure 7. Flow cytometry analysis on hepatic non parenchymal cells confirmed the presence of GFP+ cells in the liver of all treated mice, with higher percentage of GFP+ cells in FLE11-E13+BU mice. Flow cytometry analysis showed that GFP+ cells were mainly hematopoietic cells (CD45+), while only a low percentage of GFP+ cells were LSEC (CD146+CD309+).
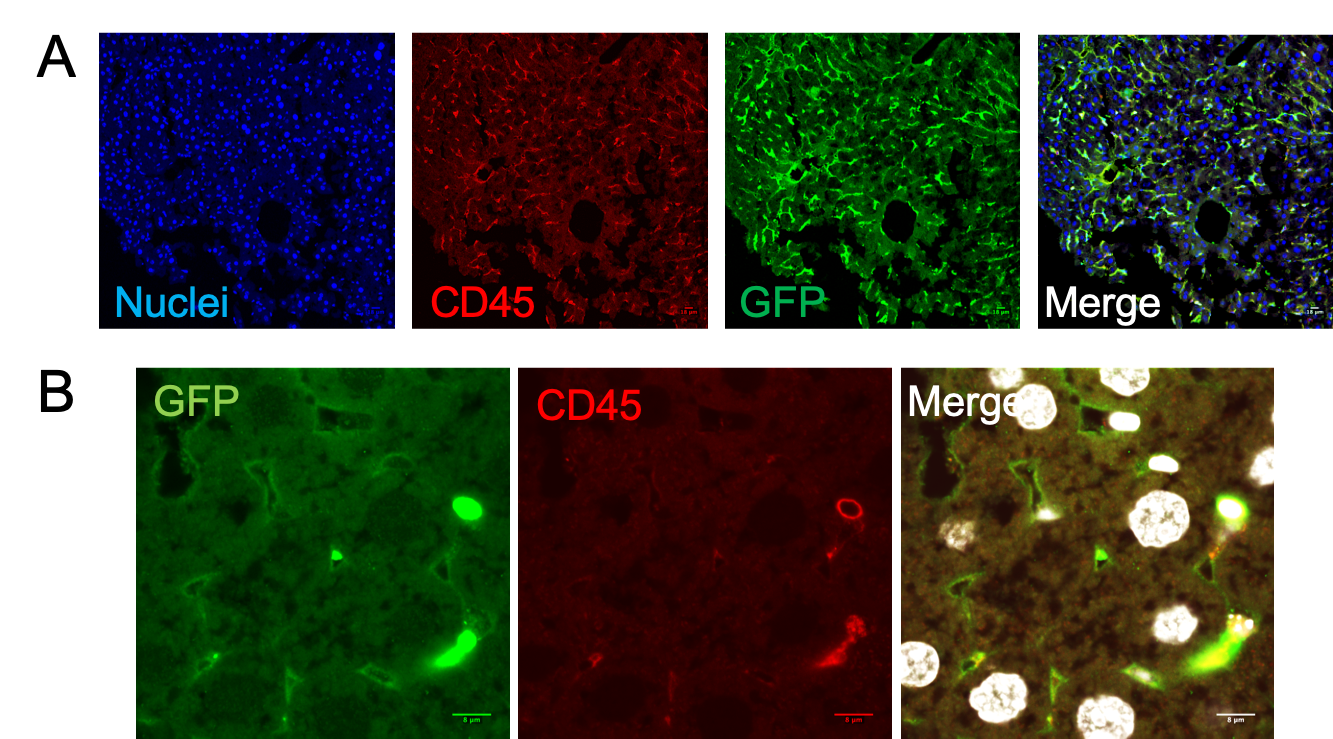
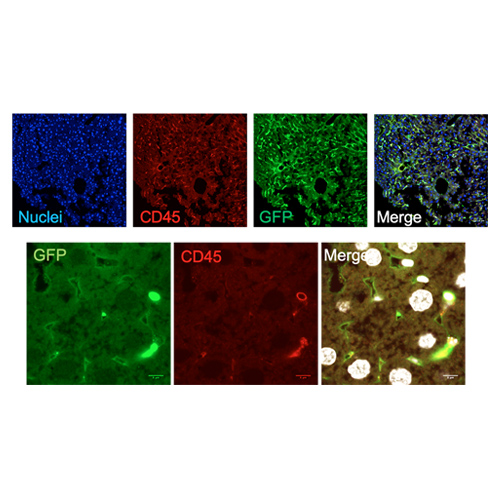
Figure 8. Immunofluorescence on liver sections from FLE11-E13+BU mice confirmed that GFP+ cells were mainly hematopietic (CD45+) cells (A). Analysis at higher magnification displayed the presence of GFP+ LSEC (CD45- and lining the sinusoids) (B).
CONCLUSIONS
Transplantation of FL cells may provide a novel and highly promising neonatal preclinical model for HA treatment, paving the way for studies aiming at deriving long-term reconstituting “fetal-like” hemato/vascular progenitors from other sources (e.g. iPS).