Rossini Sofia1, Davide Matino2, Giancarlo Castaman3, Giovanni Di Minno4, Paola Giordano5, Maria Elisa Mancuso6, Emanuela Marchesini1, Flora Peyvandi6, Elena Santagostino6, Cristina Santoro7, Michele Schiavulli8, Antonella De Luca1, Marco Gargaro1, Paolo Puccetti1, Alfonso Iorio2 and Francesca Fallarino1
1Department of Experimental Medicine, University of Perugia, Perugia, Italy; 2McMaster University, Hamilton, ON, Canada; 3Careggi University Hospital, Florence, Italy; 4Federico II University, Napoli, Italy; 5University of Bari, Bari, Italy; 6Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 7Hematology, University Hospital Policlinico Umberto I, Rome, Italy; 8Santobono – Pausilipon Hospital, Napoli, Italy
BACKGROUND AND AIMS
The development of an immunogenic response to therapeutic FVIII, with the appearance of neutralizing antibodies (“inhibitors”), is the main adverse event in hemophilia A treatment. The only proven and currently available strategy to eliminate inhibitors is ITI, based on the long-term, intravenous administration of high-dose FVIII. The success rate of ITI is about 60-70 % and not much information is available about the potential factors of success and failure. The aim of our study is to investigate the immunological events that develop in these patients undergoing ITI and to understand which parameters influence the achievement of FVIII tolerance.
MATERIALS AND METHODS
This is a multicenter, prospective cohort study that enrolled 20 severe hemophilia A patients with high-titer inhibitors, candidate to ITI. Blood samples were collected at different time points and total PBMCs were cultured with the FVIII product administered during ITI. Studies of gene expression profiling and phenotypic characterization were performed using immune cells. Multiplex cytokines arrays were also conducted to measure the cytokine levels in plasma samples and in cell culture supernatants. Plasma samples were also tested for anti-FVIII antibodies determination.
RESULTS
We report the results of 7 patients that completed the ITI treatment, 3 of them successfully achieved tolerance while 4 failed to eradicate inhibitors. We found a sustained IgG4 anti-FVIII response, a production of pro-inflammatory cytokines and a consistent immune gene expression in patients who failed to induce FVIII-tolerance during the ITI. Notably, successfully tolerized patients experienced a decreasing anti-FVIII IgG4 response and an increased production of regulatory cytokines, such as TGF-β.
- Decreasing anti-FVIII IgG4 response in cases of success (PZ 1, 2, 3);
- Sustained anti-FVIII IgG4 response during the entire treatment in cases of failure (PZ 4, 5, 6, 7).
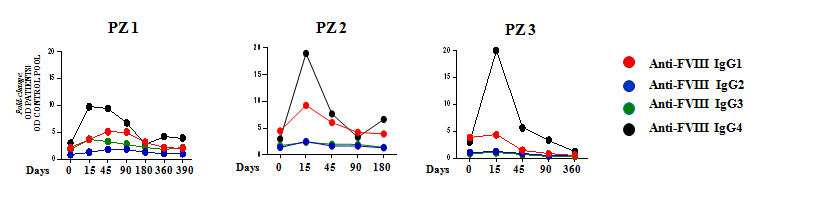
Kinetics representation of IgG subclasses plasmatic level in success cases. Antibody titres at each time point were determined by ELISA test in plasma samples. Data are expressed as fold-change, comparing the OD of patient plasma and the OD of a standard control pool plasma.
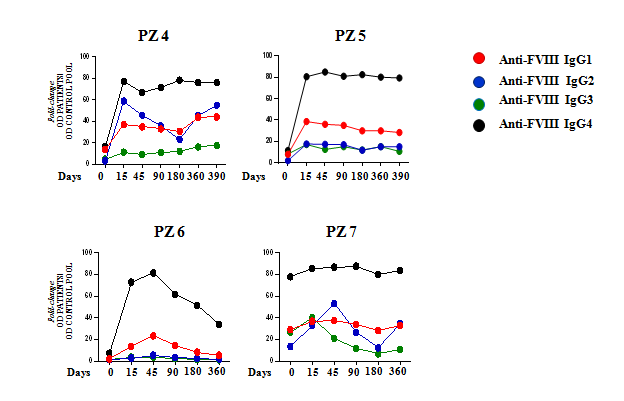
Kinetics representation of IgG subclasses plasmatic level in failure cases. Antibody titres at each time point were determined by ELISA test in plasma samples. Data are expressed as fold-change, comparing the OD of patient plasma and the OD of a standard control pool plasma.
- Increased plasmatic production of TGF-β in patients that successfully achieved FVIII immune tolerance;
- No significative changes in TGF-β plasmatic level in failed patients during immune tolerance induction therapy.
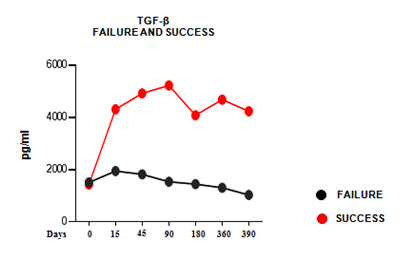
Kinetics of TGF-β levels in plasma. TGF-β concentration determined by ELISA test in plasma samples collected at each time point. Each curve was obtained using the average of TGF-β values (pg/ml), measured for each patient at each time point, and is representative of each group of patients.
CONCLUSIONS
This ongoing study will provide us with more knowledge about potential biomarkers of success or failure and about which immune-regulatory mechanisms are missing in failure’s cases.
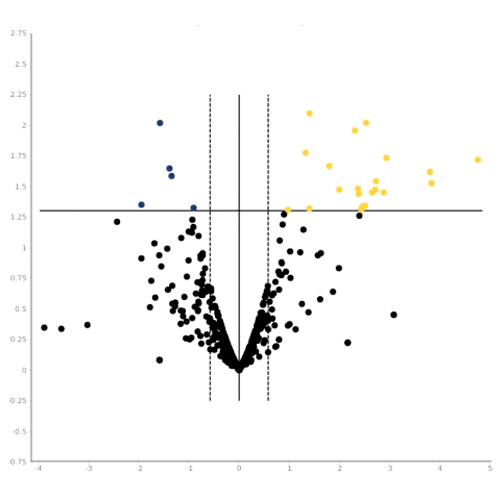
- Immune gene expression analysis performed in failed cases;
- Over-expression of genes coding for pro-inflammatory cytokines, chemokines and growth factors;
- Down-regulation of the gene coding for the immunomodulatory enzime IDO1 (Indoleamine 2,3-dioxygenase).
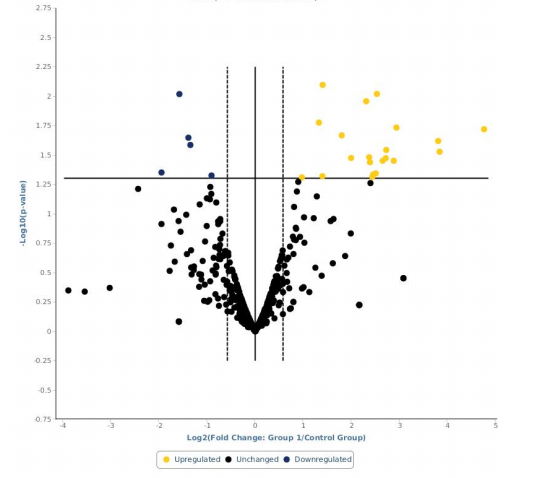
Final Report Failure. Gene expression profiling analysis for patients who failed to achieve the FVIII-immune tolerance. Data expressed as fold-change, comparing the gene expression profile in the treated and in the control groups.