L.H. Bukkems1*, J.M. Heijdra2*, M. Mathias3, P.W. Collins4, C.R.M. Hay5, R.C. Tait6, S. Mangles7, B. Myers8, G. Evans9, B. Bailiff10, N. Curry11, J. Payne12, S. Austin13, M.C.H.J. Goedhart2, F.W.G. Leebeek14, K. Meijer15, K. Fijnvandraat16, P. Chowdary17**, R.A.A. Mathôt1**, M.H. Cnossen2** for UK-EHL Outcomes Registry and OPTI-CLOT collaboration
1Hospital Pharmacy-Clinical Pharmacology, Amsterdam University Medical Centers, Amsterdam, the Netherlands; 2Department of Pediatric Hematology, Erasmus University Medical Center – Sophia Children’s Hospital Rotterdam, Rotterdam, the Netherlands; 3Haemophilia Comprehensive Care Centre, Great Ormond Street Hospital for Children NHS Foundation Trust, London, UK; 4Arthur Bloom Haemophilia Centre, School of Medicine, Cardiff University Hospital, Cardiff, UK; 5University Department of Haematology, Manchester University NHS Foundation Trust, Manchester, UK; 6Department of Haematology, Royal Infirmary, Glasgow, UK; 7Haemophilia, Haemostasis and Thrombosis Centre, Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK; 8Department of Haematology, United Lincolnshire Hospitals NHS Trust, Lincoln, UK; 9Department of Haematology, East Kent Hospitals University NHS Foundation Trust, Kent, UK; 10Department Haematology and Blood Transfusion, University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK; 11 Oxford Haemophilia and Thrombosis Centre and Oxford NIHR BRC, Churchill Hospital, Oxford, UK; 12Department of Paediatric Haematology, Sheffield Children’s NHS Foundation Trust, Sheffield, UK; 13 Centre for Haemostasis and Thrombosis, St George’s University Hospitals NHS Foundation Trust, London, UK; 14Department of Hematology, Erasmus University Medical Center, Rotterdam, The Netherlands; 15Department of Hematology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands; 16Department of Pediatrics, Amsterdam University Medical Centers – Emma Children’s Hospital, The Netherlands; 17Katharine Dormandy Haemophilia Centre and Thrombosis Unit, Royal Free London NHS Foundation Trust, London, UK. *Shared first and last ** authorships
Background
- Pharmacokinetic(PK)-guided dosing can aid in individualizing replacement therapy for hemophilia patients.
- The current population PK models used for PK guided treatment are based on clinical trial data, whereas real clinical patients may differ significantly from carefully selected clinical trial populations.
- The aim of this study was to validate and potentially optimize a previously published rFVIIIFc population PK model [1] with independent real world clinical data.
Methods
Clinical data of hemophilia A patients treated with rFVIIIFc concentrate participating in the United Kingdom Extended Half-Life Outcomes Registry were collected from eight different UK treatment centers. FVIII levels were determined by one-stage or/and chromogenic assay. The predictive performance of the previously published model was assessed using mean percentage error (MPE, bias; equation 1) and mean absolute percentage error (MAPE, inaccuracy; equation 2) and by visual inspection of the goodness-of-fit plots.
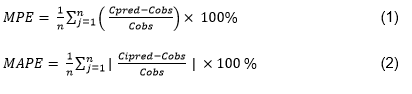
Evaluation of the predictive performance of the model in more detail was done by plotting patient characteristics such as age and body weight against the inter-individual variability (ETA, ƞ) in the PK parameters clearance and central volume of distribution. An alternative extended population PK model was developed using nonlinear mixed-effects modelling (NONMEM). At last, a clinical case of the pediatric hematology department was used to demonstrate the difference between the published and the optimized population PK model.
Results
Data of 43 hemophilia A patients (baseline FVIII ≤2 IU/dL), aged 5 to 70 years, were collected. The model could predict the 244 rFVIII-FC concentrations without significant bias 2.9 ± 9.8% and with sufficient accuracy (15.8 ± 6.1 %). However, when analyzing data in more detail, clearance and central distribution volume were under predicted in patients <12 years (figure 1). An alternative model was created by modifying the relationship between bodyweight and both clearance and central volume and by re-estimating the PK parameters (table 1). This new alternative model was internally validated with among others a visual predictive check (figure 2) and its importance was underlined by a clinical patient of 2 years, as the alternative model showed most reliable results in determing the PK profile of this hemophilia A patient (figure 3).
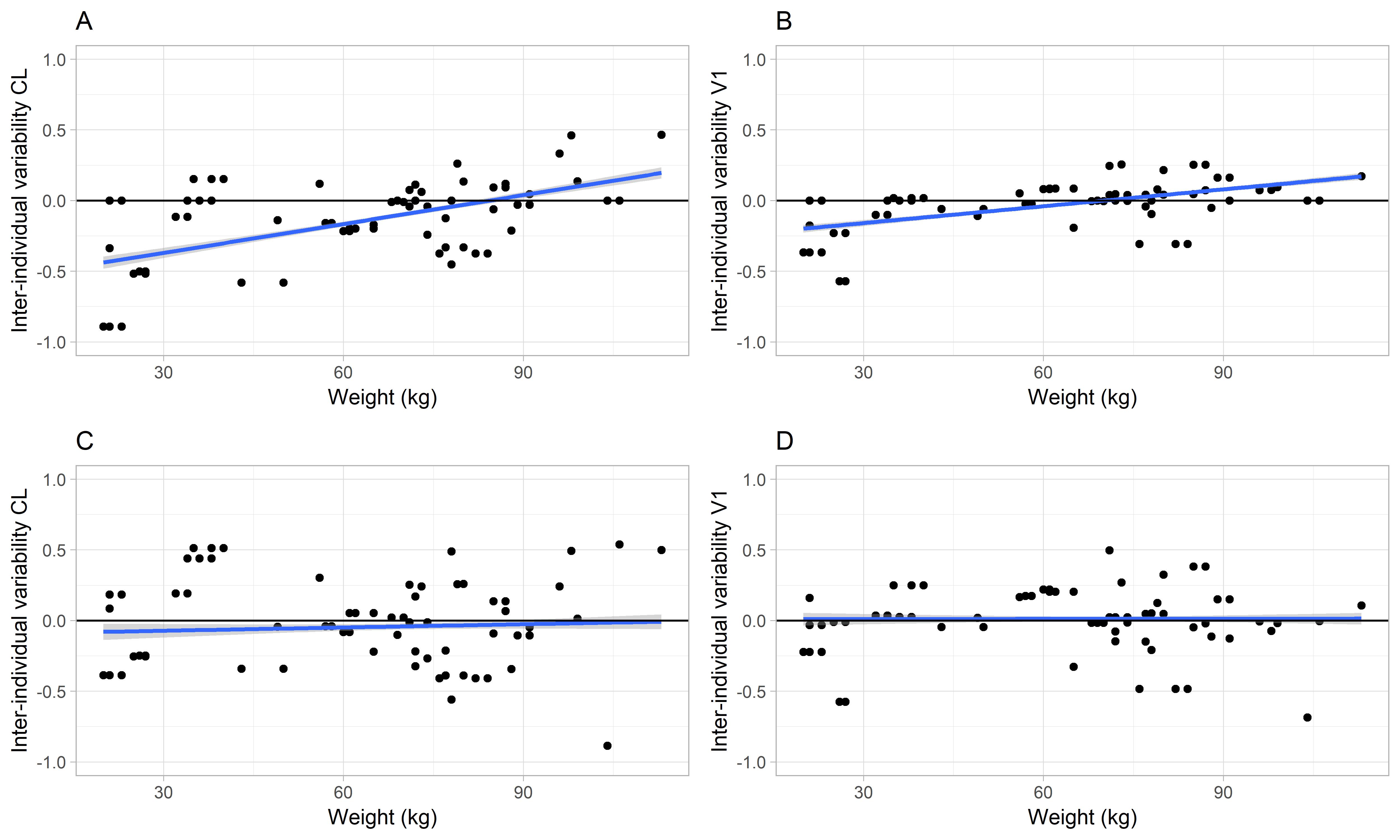
Figure 1: Inter-individual variability of (A) clearance or (B) central volume of distribution versus body weight using the earlier published model. Corresponding plots C and D present the inter-individual variability for the developed population PK rFVIII-Fc model. The individual data (black circles) are visualized as a trend line (blue solid line) that approximates the line of identity (black solid line). With the introduction of weight as covariate on clearance in the new model a less biased estimation is obtained at lower weight.
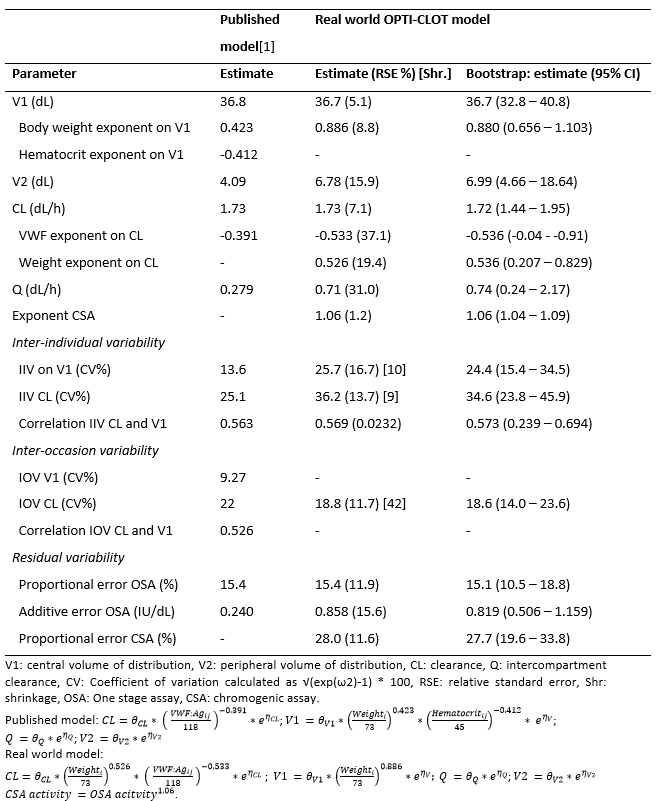
Table 1: Pharmacokinetic (PK) parameter estimates of the previously published and real world population PK rFVIII-Fc models
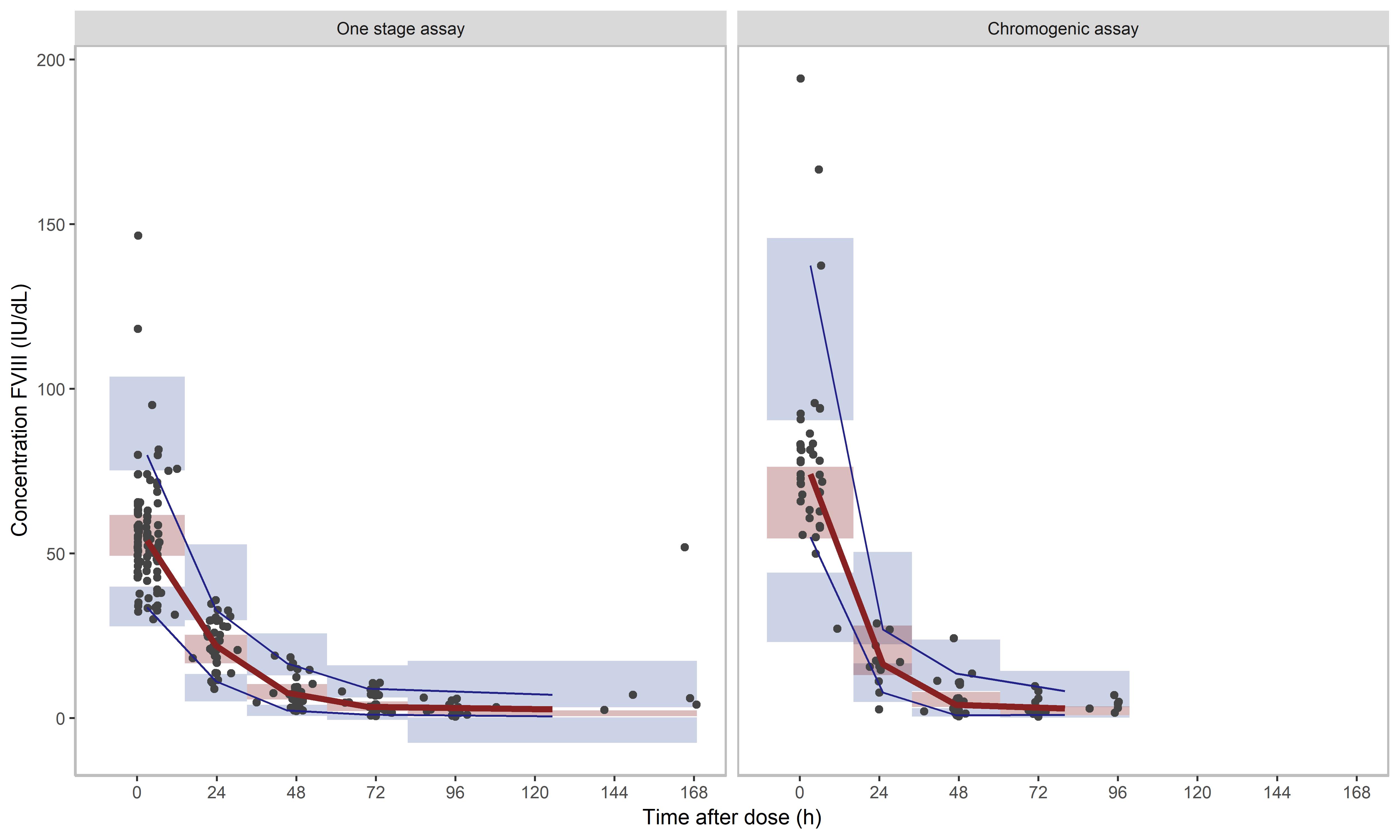
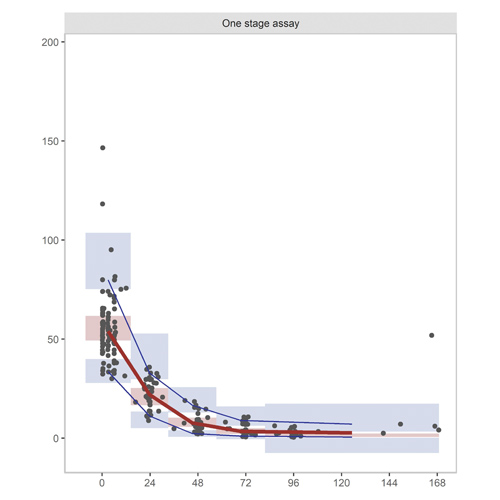
Figure 2: Prediction-corrected visual predictive check (VPC) of (left) real world rFVIII-Fc model for one stage assay (OSA) samples and (right) chromogenic assay (CSA) samples. The median (red line) and 95% CI (blue lines) of the observed data (black dots) are plotted against the simulated data (n=1000) indicated as highlighted areas: the red box being the median and the blue box the 95% prediction interval. A model predicts the concentrations adequately when the blue and red lines run through the corresponding boxes.
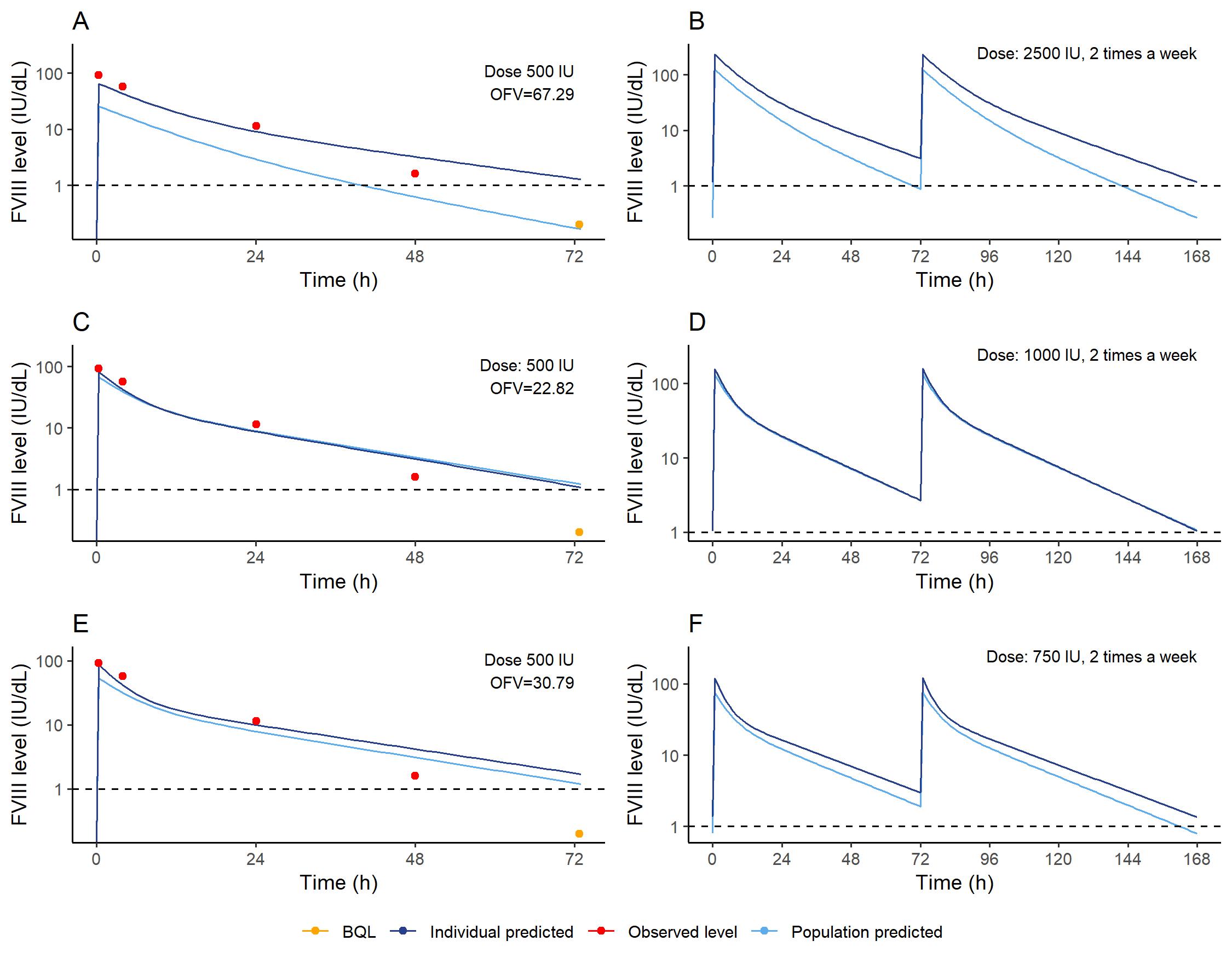
Figure 3: PK profile of the clinical case using the (A) previously published, (C) real world rFVIII-Fc PK model and (E) without correction for chromogenic assay (CSA) method. Based on the PK profile of the (B) previously published, (D) real world rFVIII-Fc PK model with correction for CSA samples and (E) without correction for CSA method, the required dosing schemes to maintain FVIII levels >1 IU/dL (dashed line) with two times a week dosing are drafted for this patient. The objective function values (OFV) of the fits are presented in the top right corners of the PK profiles. The PK profile defined using the new alternative model with adequate correction for CSA samples could describe as well as the top as the trough levels the most adequate, and this dosing scheme is therefore regarded as most trustworthy.
Conclusions
The published rFVIIIFc model was developed for patients ≥12 years. Our study demonstrated that, this model will give erroneous results when applied in patients younger than 12 years. The developed alternative model allowed a better description for younger patients as well. This study demonstrates the importance of validating population PK models used in clinical routine.
Literature
[1] Nestorov I, Neelakantan S, Ludden TM, et al. Population pharmacokinetics of recombinant factor VIII Fc fusion protein. Clin Pharmacol Drug Dev 2015; 4: 163–174.
Contact information:
Laura Bukkems
l.h.bukkems@amc.uva.nl
Hospital Pharmacy-Clinical Pharmacology
Amsterdam University Medical Centers, Amsterdam, the Netherlands