R.J. Liesner1, M. Jansen2, S. Knaub3
1Great Ormond Hospital for Children NHS Trust Haemophilia Centre, London, UK; 2Octapharma Pharmazeutika Produktionsges.mbH, Vienna, Austria; 3Octapharma AG, Lachen, Switzerland
Introduction
- Neutralising antibodies against exogenous clotting factor VIII (FVIII) remain the most significant treatment complication in patients with haemophilia A, particularly in previously untreated patients (PUPs)1-4
- Inhibitors develop in approximately 35% of PUPs with severe haemophilia A5,6
- Inhibitors in PUPs usually develop within the first 20 exposure days (EDs)7
- Approximately 70% of inhibitors in PUPs are high-titre ( ≥ 5 BU/mL)5,6
- Several risk factors for inhibitor development variables have been identified, including family history, ethnicity, specific F8 gene mutations, product type and treatment intensity7,8
- In the SIPPET study6 – a prospective, randomised, controlled study in PUPs – the cumulative rates of inhibitor development were
- 26.8% (18.6% high-titre) in patients treated with plasma-derived FVIII products containing von Willebrand factor (pdFVIII/VWF)
- 44.5% (28.4% high-titre) in patients treated with recombinant FVIII (rFVIII) products derived from hamster cell lines
- A post-hoc analysis of SIPPET data9 revealed that in patients with non-null F8 mutations (some level of functional protein remaining), no inhibitors developed in patients receiving pdFVIII/VWF treatment, whereas the cumulative incidence of inhibitors was 43% in patients treated with rFVIII derived from hamster cell lines
- Nuwiq® (simoctocog alfa) is a 4th generation rFVIII product produced in a human cell line, without chemical modification or protein fusion10
- The efficacy and safety of Nuwiq® were demonstrated in studies of previously treated patients with severe haemophilia A, including 59 children under 12 years of age10
- The NuProtect study was initiated in 2013 to assess the immunogenicity, efficacy and safety of Nuwiq® in PUPs
Patients and methods
- NuProtect was a prospective, multicentre, multinational, open-label, non-controlled, phase III study
- True PUPs (no previous FVIII treatment) with severe haemophilia A of any age and ethnicity were enrolled and treated for 100 EDs or a maximum of 5 years with Nuwiq®
- Patients received Nuwiq® for prophylaxis or on-demand treatment, for treatment of breakthrough bleeding episodes (BEs) during prophylaxis and for surgical prophylaxis
- The type of treatment and dose were determined by the investigator
- Inhibitor screening by the modified Bethesda assay was performed at screening, every 3–4 EDs until ED20, then every 10–12 EDs or at least every 3 months, at completion visit, and if inhibitor development was suspected
- Inhibitor titres of ≥ 0.6 to < 5 BU/mL were defined as low-titre and ≥ 5 BU/mL as high-titre (based on peak titre)
- Cumulative inhibitor incidence (primary endpoint) and 95% confidence intervals (CIs) were calculated using Kaplan-Meier methods
Results
- The study was conducted at 38 sites in 17 countries
- 110 patients were enrolled and 108 treated with Nuwiq®
- 105 patients had at least one inhibitor test after ED1
- Median (range) age at ED1 was 12.0 (0–146) months; 82 (75.9%) patients were aged < 24 months
- The majority of patients had null (non-functional) F8 mutations (90 of 102 patients with available data [88.2%]) (Figure 1)
- 13 of 42 (31.0%) patients with a family history of haemophilia A had a family history of inhibitors
- Patients were treated for a median of 100 EDs (range 1–120), with 91 patients treated for at least 100 EDs (or until inhibitor development)
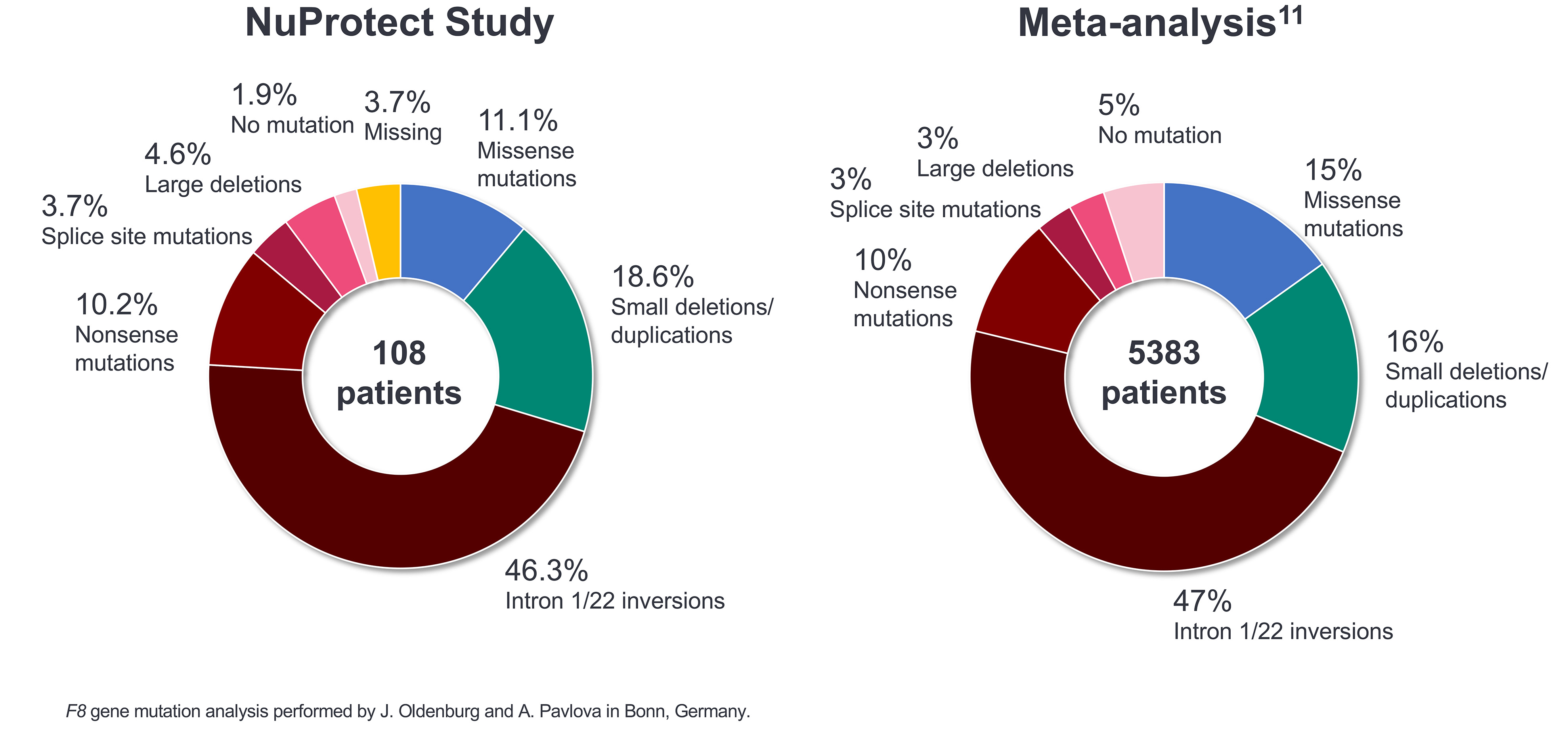
Figure 1: F8 gene mutation analysis: NuProtect vs a general severe haemophilia A population
Inhibitor development
- The cumulative inhibitor incidence (Figure 2) was
- 17.6% (95% CI: 10.0%, 25.3%) for high-titre inhibitors
- 12.3% (95% CI: 5.5%, 19.2%) for low-titre inhibitors
- 27.9% (95% CI: 19.1%, 36.7%) for all inhibitors
- Cumulative high-titre inhibitor incidence is shown alongside SIPPET6 data in Figure 3.
- The median (range) time to inhibitor development was
- 9.0 (4–24) EDs for high-titre inhibitors
- 12.0 (6–34) EDs for low-titre inhibitors
- 11.0 (4–34) EDs for all inhibitors
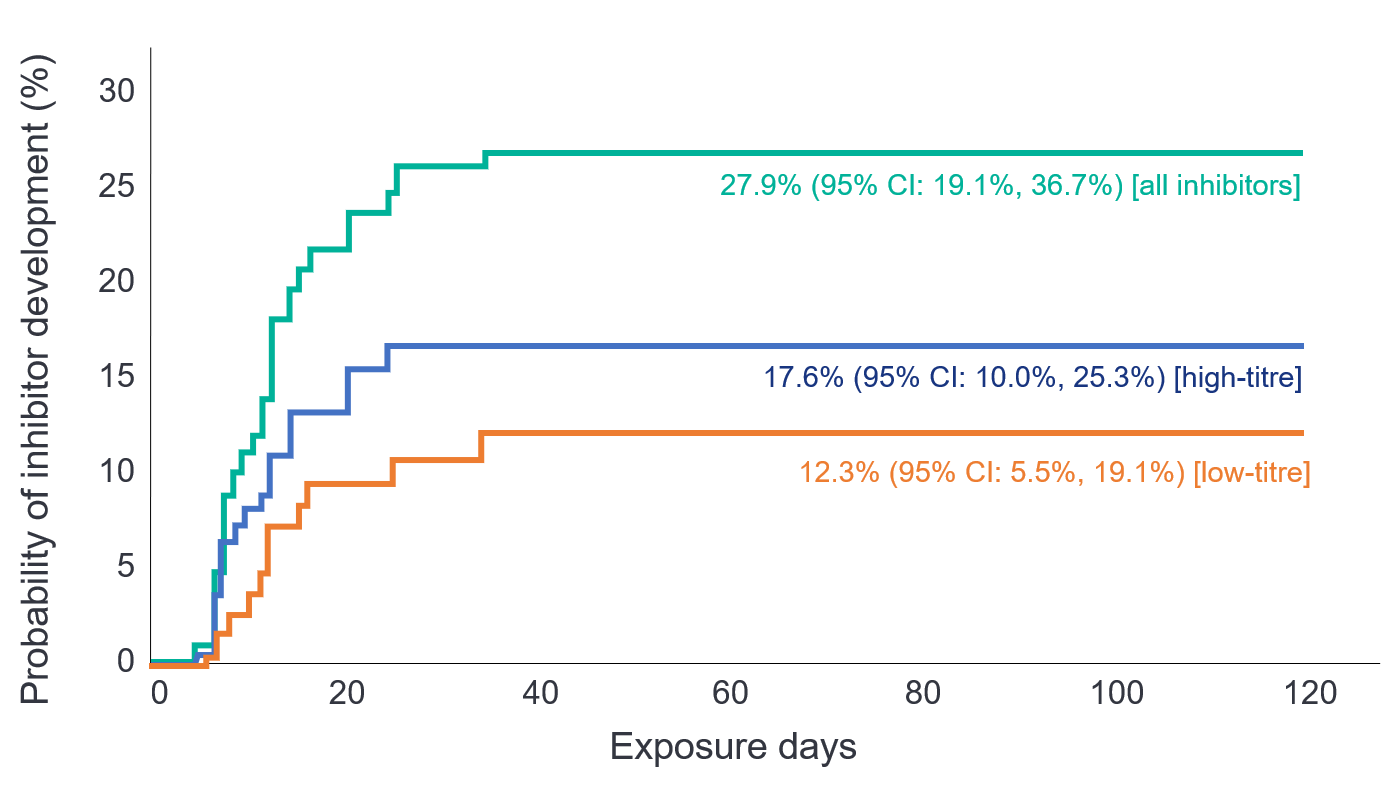
Figure 2: Cumulative inhibitor risk in PUPs treated with Nuwiq®

Figure 3: Cumulative incidence of high-titre inhibitors in SIPPET and NuProtect
- Of 90 patients with null F8 mutations, 27 (30.0%, 95% CI: 20.8%, 40.6%) developed inhibitors
- No patients with a non-null mutation developed inhibitors (0%; N=12; 95% CI: 0.0%, 26.5%) (Figure 4);
- Data are shown together with corresponding SIPPET9 data in Table 1
- In SIPPET9, 43% of patients with a non-null F8 mutation treated with hamster cell line-derived rFVIII developed inhibitors
- None of the patients with a non-null F8 mutation developed an inhibitor when treated with pdFVIII/VWF in SIPPET9 or with Nuwiq® in NuProtect
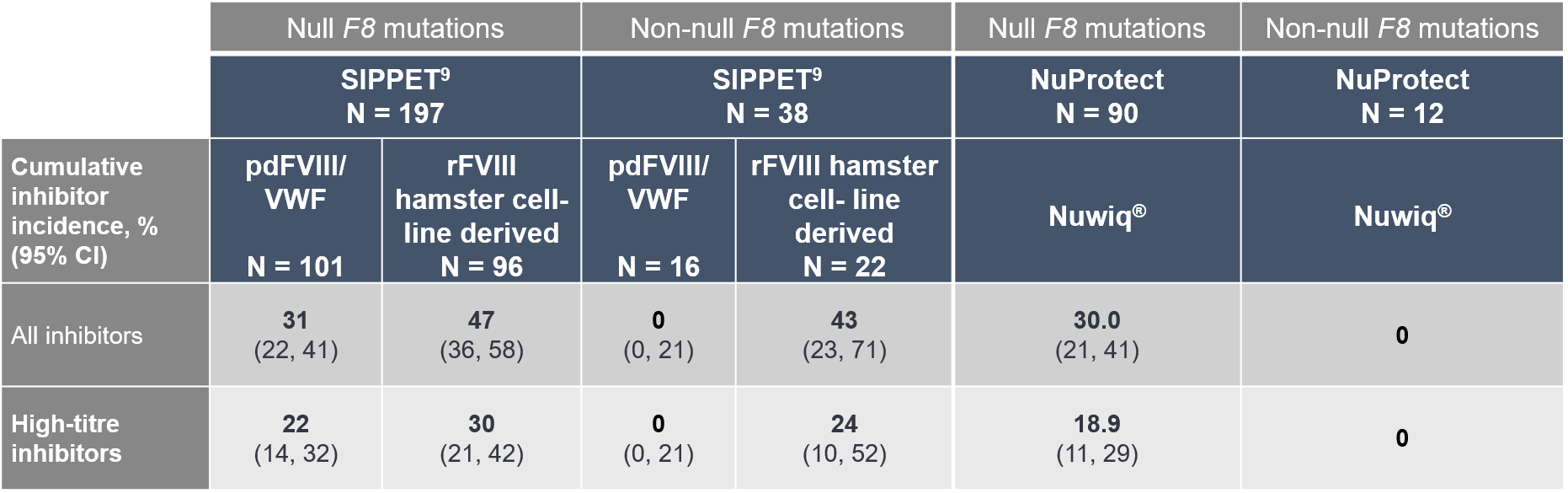
Table 1: Risk of inhibitor development by F8 mutation status in the SIPPET and NuProtect studies
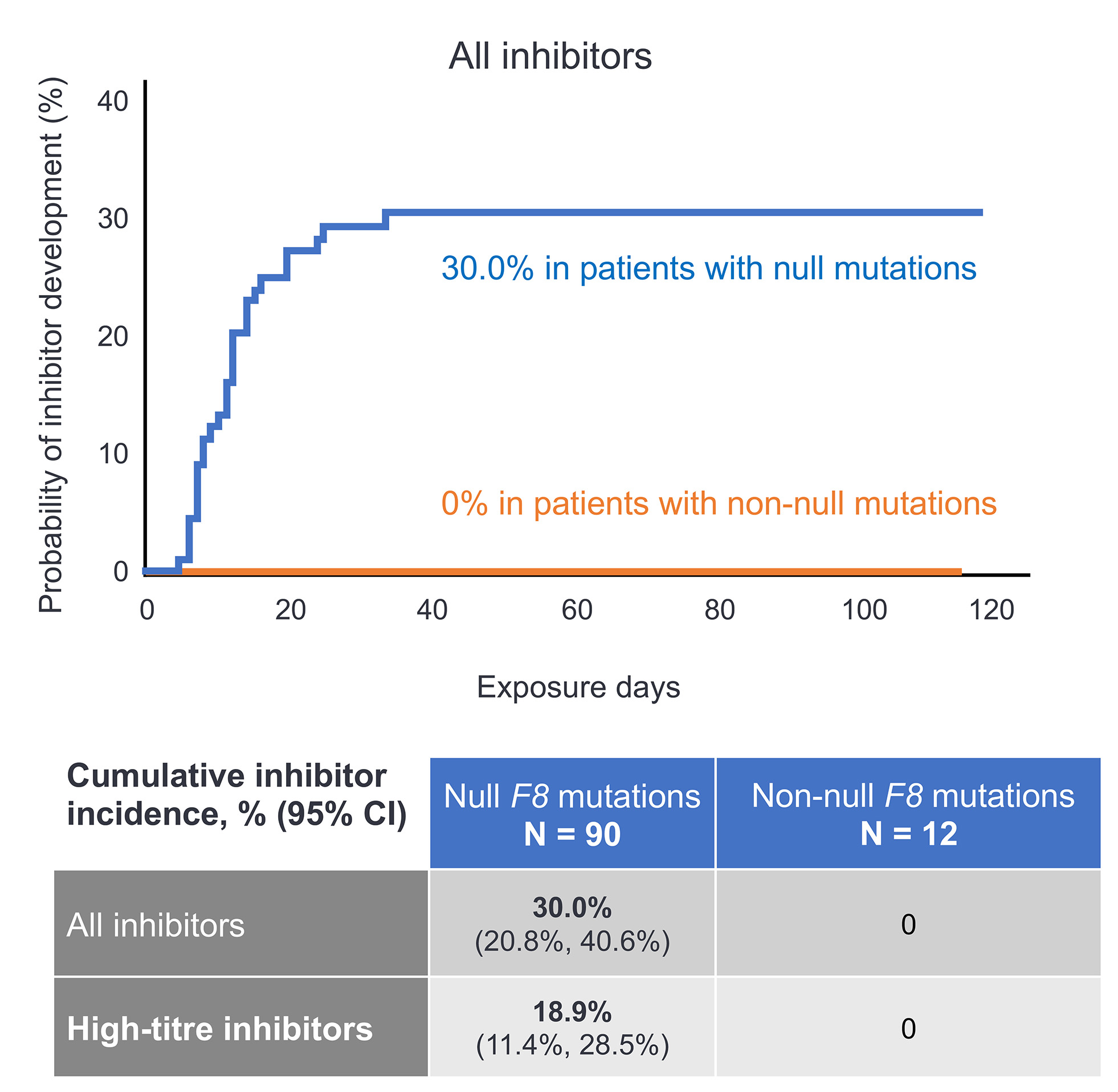
Figure 4: Cumulative inhibitor risk in PUPs treated with Nuwiq® according to F8 gene mutation
Safety
- 88 serious adverse events (SAEs) were documented in 48 (44.4%) patients
- At least possibly related SAEs, according to the investigator’s assessment, were reported in 29 patients
- FVIII inhibition (28 patients)
- Rash (1 patient, severity mild, required hospitalisation, resolved)
Conclusions
- In patients treated with Nuwiq®, the cumulative incidence of high-titre inhibitors was 17.6% and cumulative incidence of all inhibitors was 27.9%
- The cumulative incidence of high-titre and all inhibitors with Nuwiq® is lower than that for rFVIII from hamster cell lines in the SIPPET study6 (28.4% and 44.5%, respectively)
- PUPs with non-null F8 mutations did not develop inhibitors with Nuwiq® from a human cell line, as was the case for pdFVIII/VWF in the SIPPET study6, but did develop inhibitors with rFVIII from hamster cell lines in the SIPPET study9
- Nuwiq® exhibits an immunogenicity profile similar to native pdFVIII/VWF in the SIPPET study6
- Nuwiq® was well tolerated
References
- Darby SC et al. J Thromb Haemost 2004; 2:1047-54.
- Fischer K et al. Haemophilia 2005; 11:43-8.
- Di Minno M et al. Haemophilia 2010; 16:e190-201.
- Walsh CE et al. Thromb Haemost 2016; 116(Suppl. 1): S10-17.
- Gouw SC et al. N Engl J Med 2013; 368:231-9.
- Peyvandi F et al. N Engl J Med 2016; 374:2054-64.
- Goudemand J et al. Thromb Haemost 2016; 116(Suppl. 1):S2-9.
- Oldenburg J et al. Haematologica 2015; 100:149-56.
- Rosendaal FR et al. Blood 2017; 130:1757-9.
- Lissitchkov T et al. Ther Adv Hematol 2019; doi:10.1177/2040620719858471.
- Gouw SC et al. Blood 2012; 119:2922-34.