M. Unterberger, L. Wagner, N.B. Binder
Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Austria
Introduction
Factor VIII activity (FVIII:C) assays of samples containing recombinant FVIII or the new bispecific antibody emicizumab (Haemlibra®) can be associated with differences in FVIII recovery in vitro between one-stage-clotting-assays and chromogenic assays. Chromogenic assays designed with human proteins, offer the possibility to measure the activity and recovery of this new bispecific antibody emicizumab in patient samples.
To monitor FVIII-INH titers with Nijmegen-Bethesda-Assays in haemophilic patients treated with emicuzumab, it was shown that only chromogenic assays with bovine proteins can be used.
Aim of this study was to investigate if chromogenic assay containing both, human and bovine proteins, can be used to measure the recovery of emicizumab in patient samples, and if the assay can be used in FVIII-INH determination by Nijmegen-Bethesda-Assay.
Materials and Methods
Plasma from Haemophilia-A patients was used for spiking with Refacto, Fanhdi and emicizumab. The FVIII activity was determined with one-stage-clotting-assay and Technochrom FVIII:C, a chromogenic assay with human FIXa and bovine FX protein. Both assays were calibrated with reference plasma prepared from normal pooled plasma.
A set of lyophilized FVIII-INH positive controls containing either 25µg/mL or 75µg/mL emicizumab were tested with the FVIII INH reagent kit based on a modified Nijmegen-Bethesda-Assay principal. Sample were tested at 1:8, 1:16 and 1:32 dilutions respectively.
The Technochrom FVIII:C chromogenic assay with human FIXa and bovine FX protein was used for determination of residual FVIII.
Results
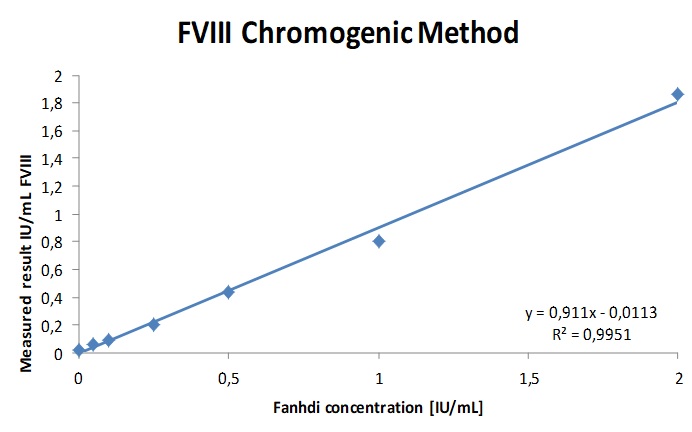
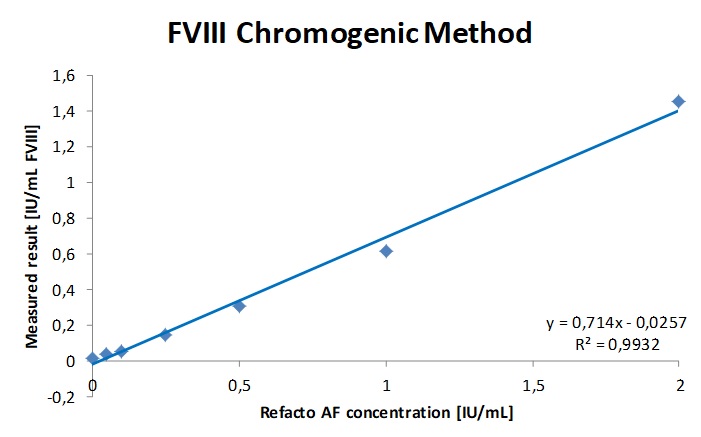
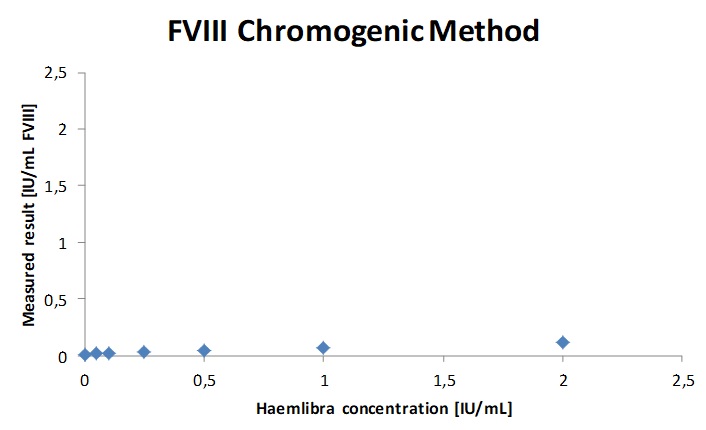
Fig. 1a. b. c. The regression parameters of the chromogenic FVIII activity determination of Haemophilia A sample spiked with increasing concentrations of Fanhdi and Refacto AF showed a good correlation with a slope of 0.91 and R2= 0.9951 for Fandhi and slope 0.71 and R2 = 0.995 for Refacto. The chromogenic FVIII assay is insensitive up to 1IU/mL (300µg/mL) emicizumab.
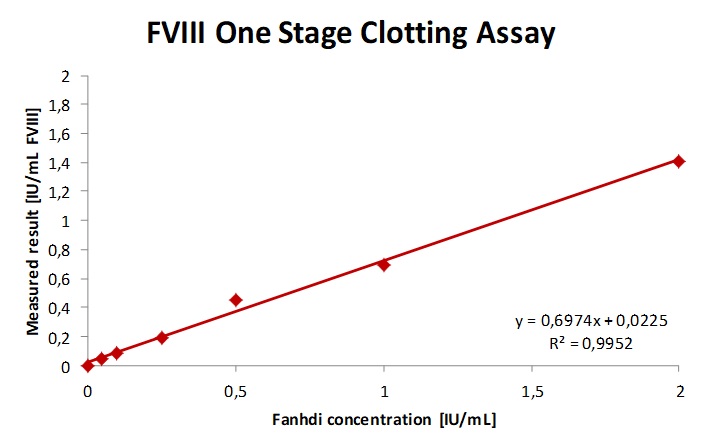
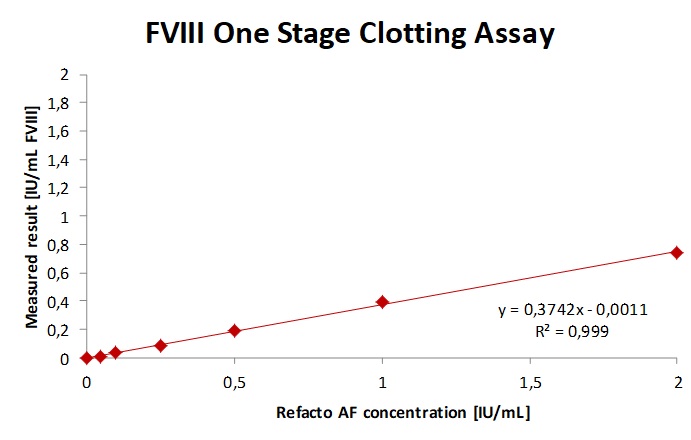
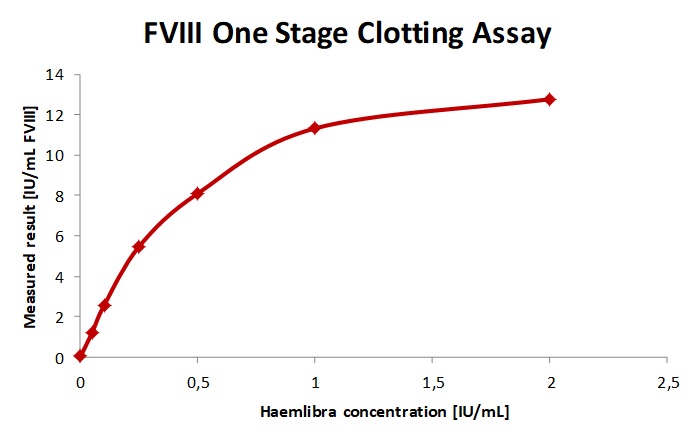
Fig. 2a. b. c The sample spiked with increasing concentrations of Fanhdi showed a slope of 0.69 and R2= 0.9952 for Fandhi. As expected the one-stage-clotting-assay underestimates Refacto AF in patient plasma, as shown by a slope of 0.4 and overestimated the sample spiked with emicizumab and are therefor not recommended.
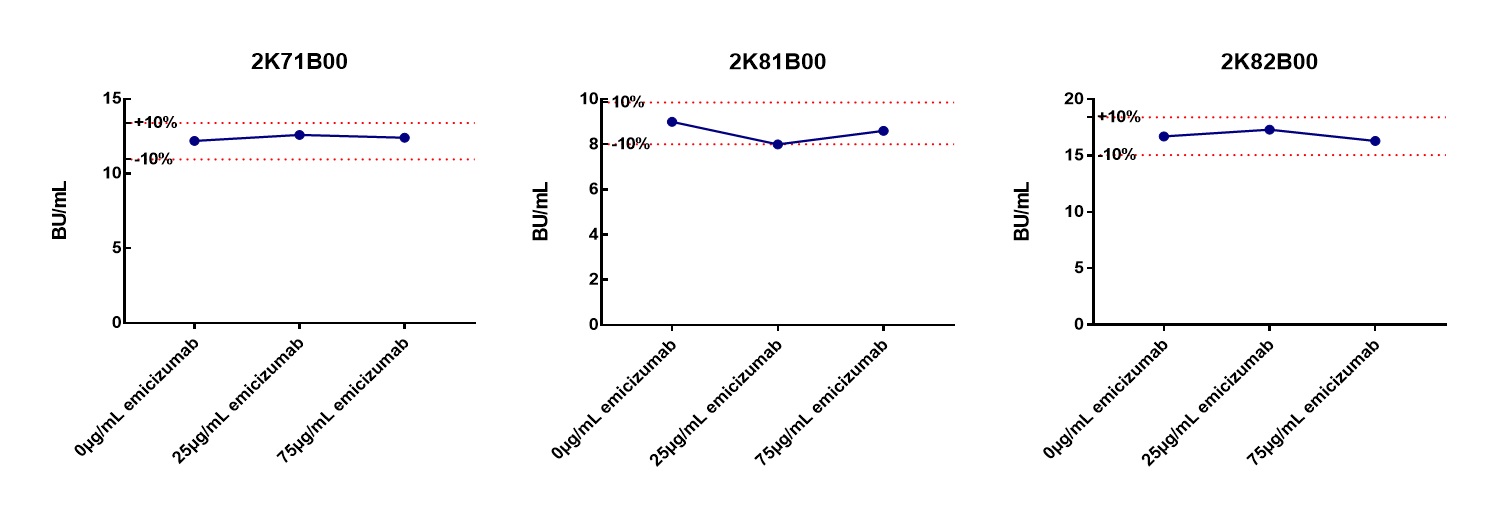
Fig. 3 FVIII-INH titer of samples spiked with emicizumab were within ±10% of the unspiked value of respective sample.
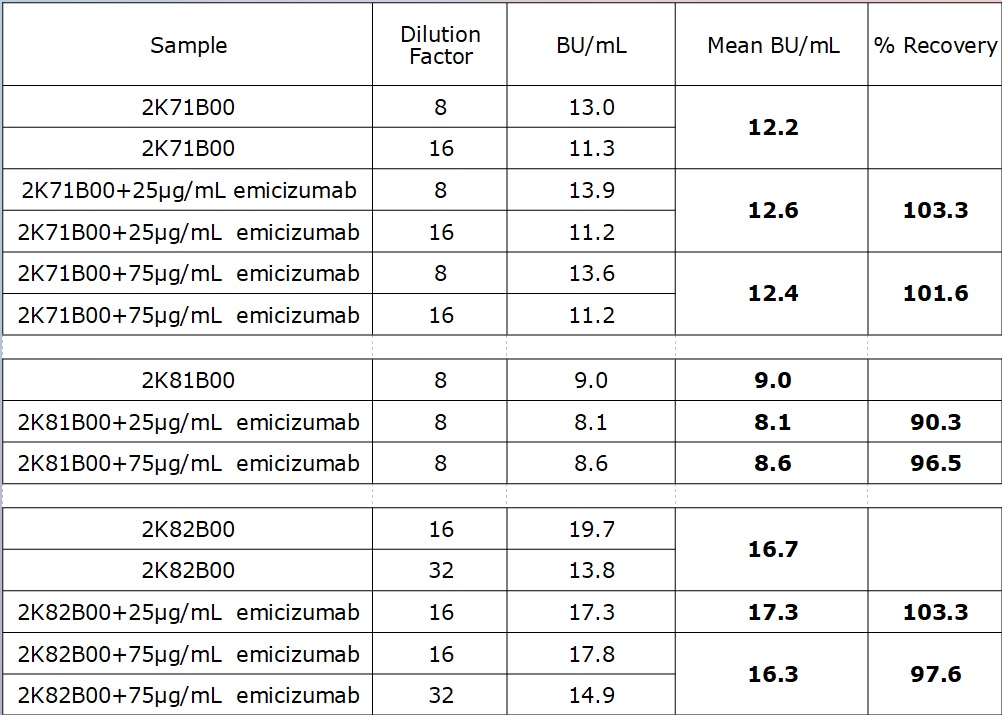
Tab. 1 Results obtained with FVIII INH reagent kit followed by chromogenic FVIII assay showed no significant sensitivity to emicizumab concentrations within the expected therapeutic range, the recovery of FVIII-INH titer for all tested samples was 100±10%.
Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH ♦ Brunner Str. 67 ♦ 1230 Vienna ♦ Austria ♦
Tel: +43 1 86 373 0 ♦ Fax: +43 1 86 373 44 ♦ Email: products@technolone.com