Carmen H Coxon1, Xinren Yu2, Scott Diamond2 Laura Diaz-Sáez3, James Beavis4, Andrew Riches1, Christina Ball1, and Sanj Raut1
1National Institute for Biological Standards and Control, Blanche Lane, South Mimms, Potters Bar, Hertfordshire, EN6 3QG, UK; 2University of Pennsylvania, 1160 Vagelos Laboratories, 3340 Smith Walk, Philadelphia, PA, 19104-6383; 3Structural Genomics Consortium, Old Road Campus, Headington, OX3 7AZ; 4Oxford Haemophilia Centre, Churchill Hospital, Oxford, OX3 7JT
Background
Congenital haemophilia A (HA) is a rare, sex-linked, hereditary condition that results in mild, moderate, or severe bleeding due to a deficiency of endogenous factor VIII (FVIII). Exogenous FVIII can be administered to restore coagulation but 30% of severe HA patients develop alloantibodies that inhibit FVIII activity (‘inhibitors’) and render treatment ineffective. As part of our ongoing work developing a reference material to standardise detection and monitoring of inhibitors, we have developed a panel of recombinant antibodies with direct research applications.
Methods
- Recombinant IgG4was expressed by ExpiCHOcells into media and purified using Protein A
- FVIII activity was functionally assessed using microplate adaptations of the one-stage clotting assay (OSCA) or chromogenic assay (CA)
- Binding was assessed by pulldown, ELISA, and surface plasmon resonance (SPR)
- For whole blood ex vivo studies, corntrypsin inhibitor (CTI)-anticoagulated whole blood was spiked with rIgG4 (blinded) at 100 nM, 30 nM, and 10 nMand then flowed over immobilisedtype I collagen and FXIa at venous shear rates (100s-1); coagulation was quantitated by platelet and fibrin deposition
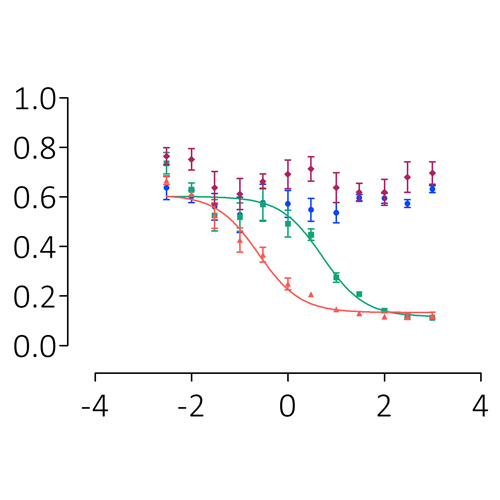
Results
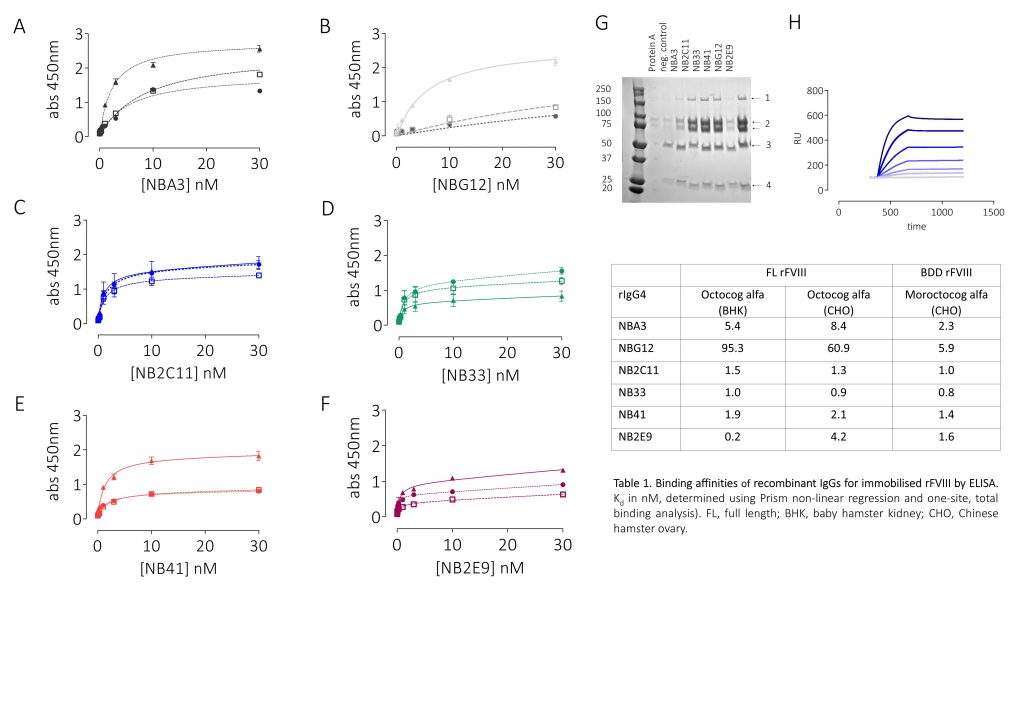
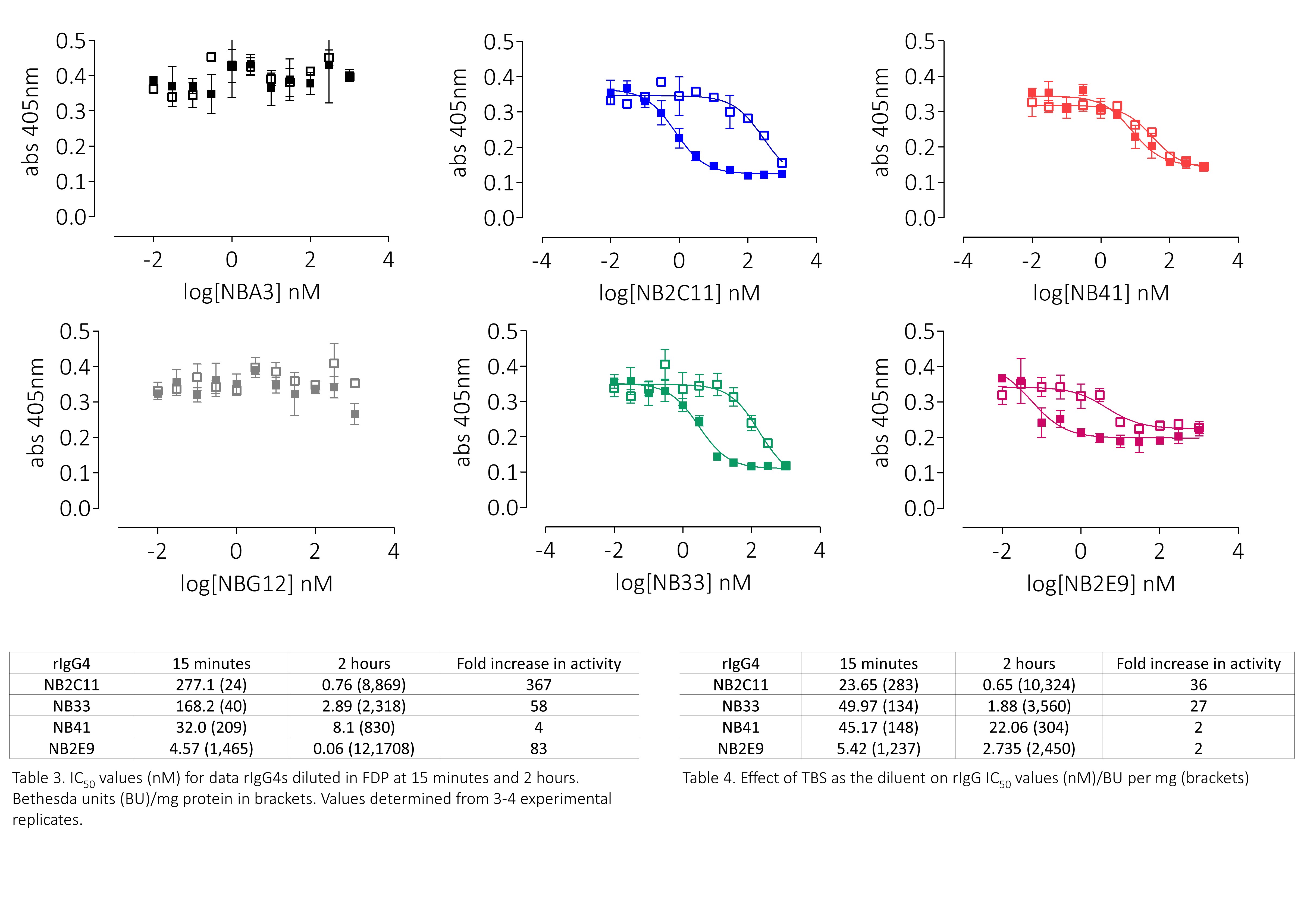
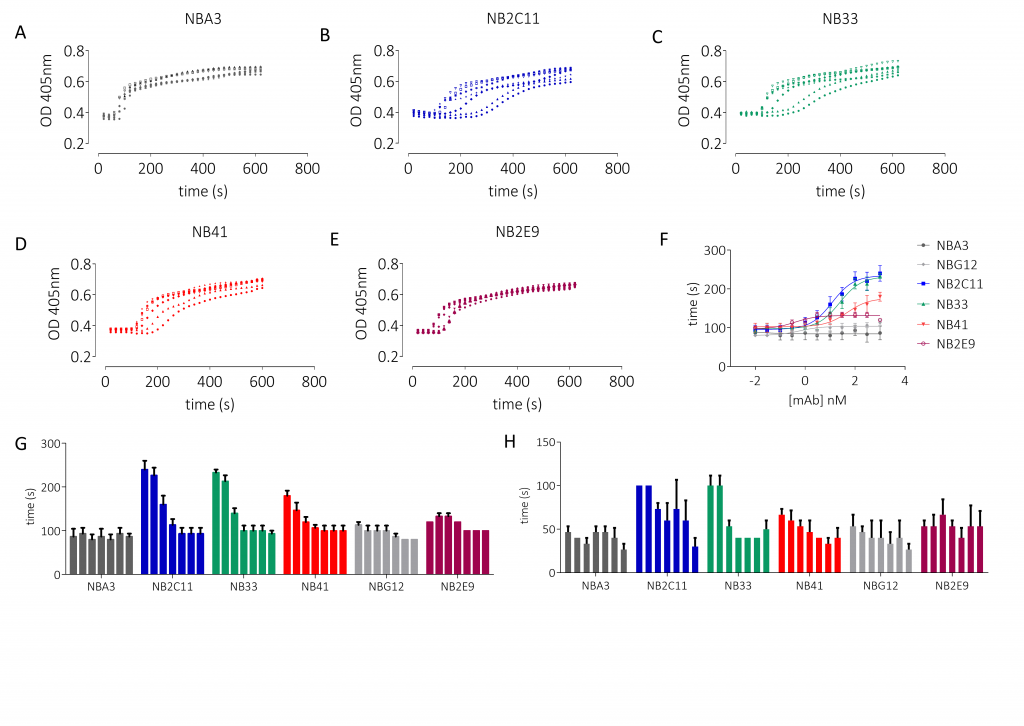
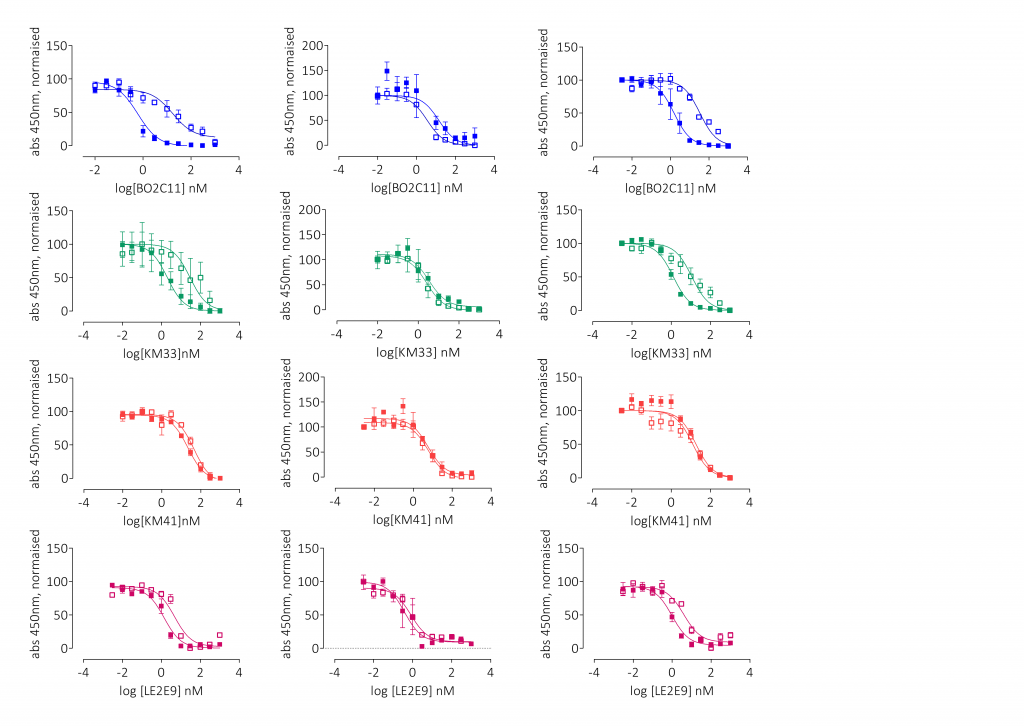
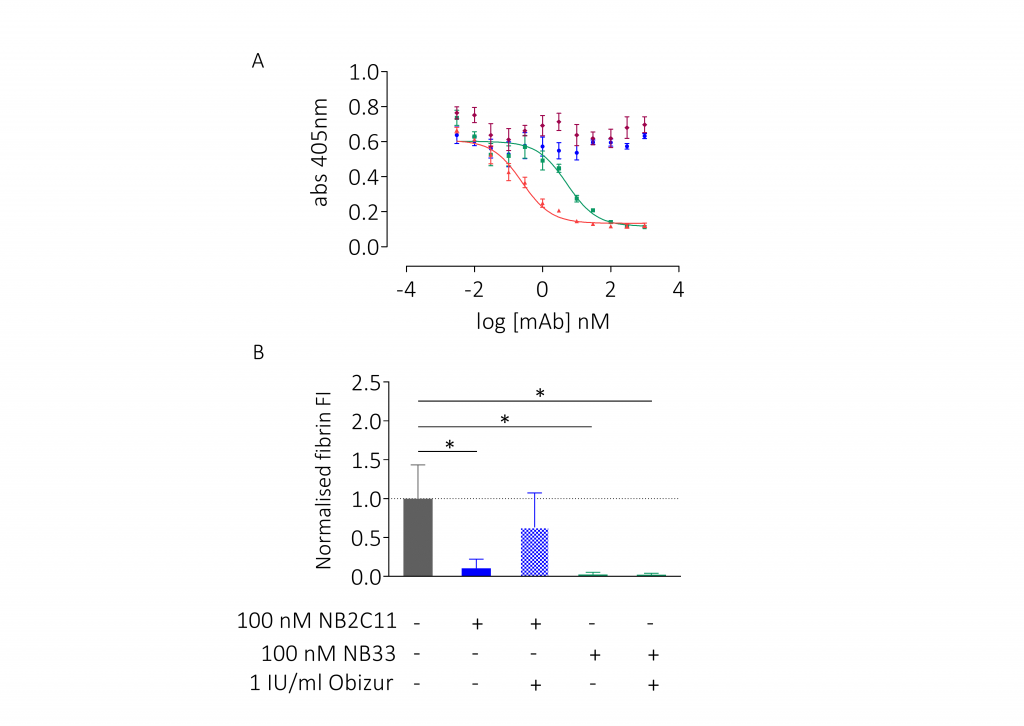
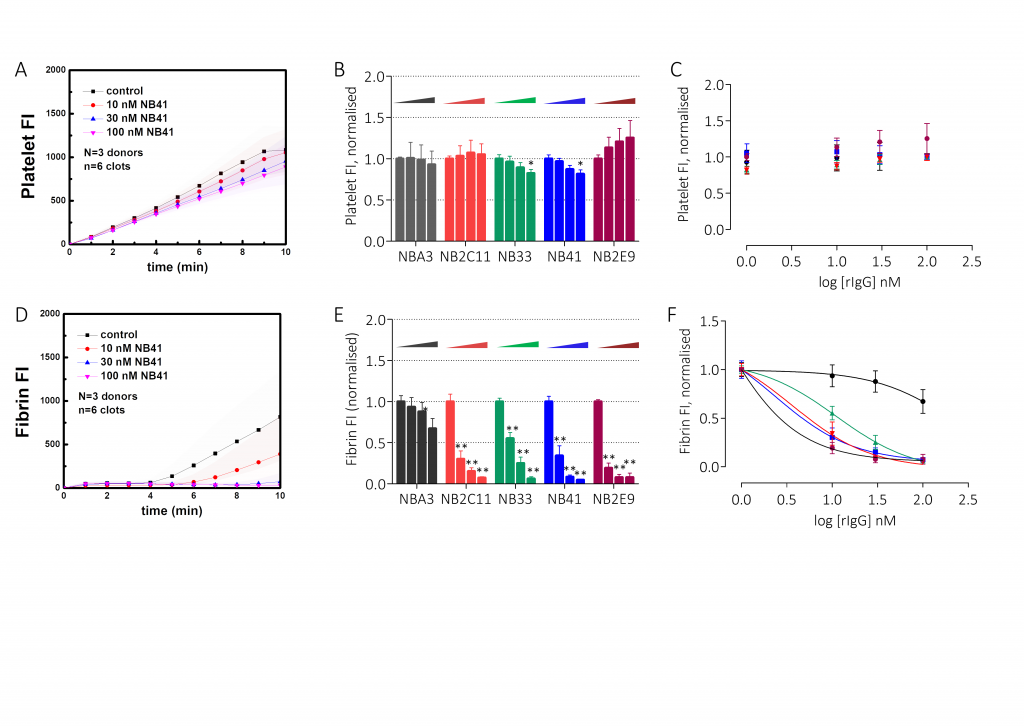
Conclusions
This panel of recombinant anti-drug antibodies contains type-1 and type-2 inhibitors with time- and concentration-dependent neutralising activity with direct research applications. These regents can also be used to test the efficacy of FVIII products in the context of inhibitor patients both in vitro and ex vivo.
REFERENCES
- Jacquemin, M.G. and J.M. Saint-Remy, Factor VIII immunogenicity. Haemophilia, 1998. 4(4): p. 552-7.
- Pratt, K, ADAs: Emerging Approaches to Predict, Reduce or Reverse Biotherapeutic Immunogenicity. Antibodies, 2018. 7(2): p. 1-18.
- Varthaman, A. and S. Lacroix-Desmazes, Pathogenic immune response to therapeutic factor VIII: exacerbated response or failed induction of tolerance? Haematologica, 2019. 104(2): p. 236-244.
- Raut, S., et al., Variability in FVIII concentrate measurement: results from SSC field collaborative studies. JTH, 2003. 1(9): p. 1927-34.
- Meijer, P. and B. Verbruggen, The between-laboratory variation of FVIII inhibitor testing: the experience of the external quality assessment program of the ECAT foundation. Semin Thromb Hemost, 2009. 35(8): p. 786-93.
- Longstaff, C. and f. subcommittee on, Development of Shiny app tools to simplify and standardize the analysis of hemostasis assay data: communication from the SSC of the ISTH. J Thromb Haemost, 2017. 15(5): p. 1044-1046.
- Shi, Q., et al., Factor VIII inhibitors: von Willebrand factor makes a difference in vitro and in vivo. JTH, 2012. 10(11): p. 2328-37.
- Dimitrov, J.D., et al., A hFVIII inhibitor modulates FVIII surface electrostatics at a VWF-binding site distant from its epitope. JTH, 2010. 8(7): p. 1524-31.
- Dasgupta, S., et al., VWF protects FVIII from endo by DCs and subsequent presentation to immune effectors. Blood, 2007. 109(2): p. 610-2.