Ri J Liesner1, Alice Wilkinson2, Mary Mathias1, Neha Bhatnagar2, Tina Biss2, Georgina W Hall2
1Haemophilia Comprehensive Care Centre, Great Ormond Street Hospital for Children, NHS Foundation Trust, London, UK; 2Children’s Hospital Oxford, Oxford Haemophilia and Thrombosis Comprehensive Care Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, UK; 3Newcastle Haemophilia Comprehensive Care Centre, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK
Introduction
- The risk of developing inhibitors to coagulation factor VIII (FVIII) remains a serious concern when treating children with haemophilia A
- Immune tolerance induction (ITI) with FVIII is the only proven approach to eradicate inhibitors1,2
- Human cell line-derived recombinant FVIII (simoctocog alfa, Nuwiq®) has demonstrated low immunogenicity in previously untreated patients (PUPs) with haemophilia A
- In the interim analysis of 66 PUPs in the NuProtect study, the cumulative incidence of high-titre inhibitors was 12.8% (95% confidence interval (CI): 4.5%, 21.2%) and the cumulative incidence of all inhibitors was 20.8% (95% CI: 10.7%, 31.0%)3
- We present the interim results of an ongoing case series from 3 centres in the UK of 10 PUPs (9 at time of abstract submission) with severe haemophilia A, who, having developed inhibitors to simoctocog alfa, received ITI using the same product
Methods
- 10 PUPs with severe haemophilia A (FVIII:C < 1) who developed inhibitors to simoctocog alfa were treated with simoctocog alfa for ITI
- ITI regimen was at the investigator’s discretion and was adjusted according to patient bleeding characteristics
- Success of ITI was assessed based on objective, defined, widely-accepted criteria as shown in Table 1
- Bleeding rate, tolerability and safety were also assessed

Table 1. Criteria for successful ITI4
Results
- Information on demographics and baseline characteristics are shown in Figure 1
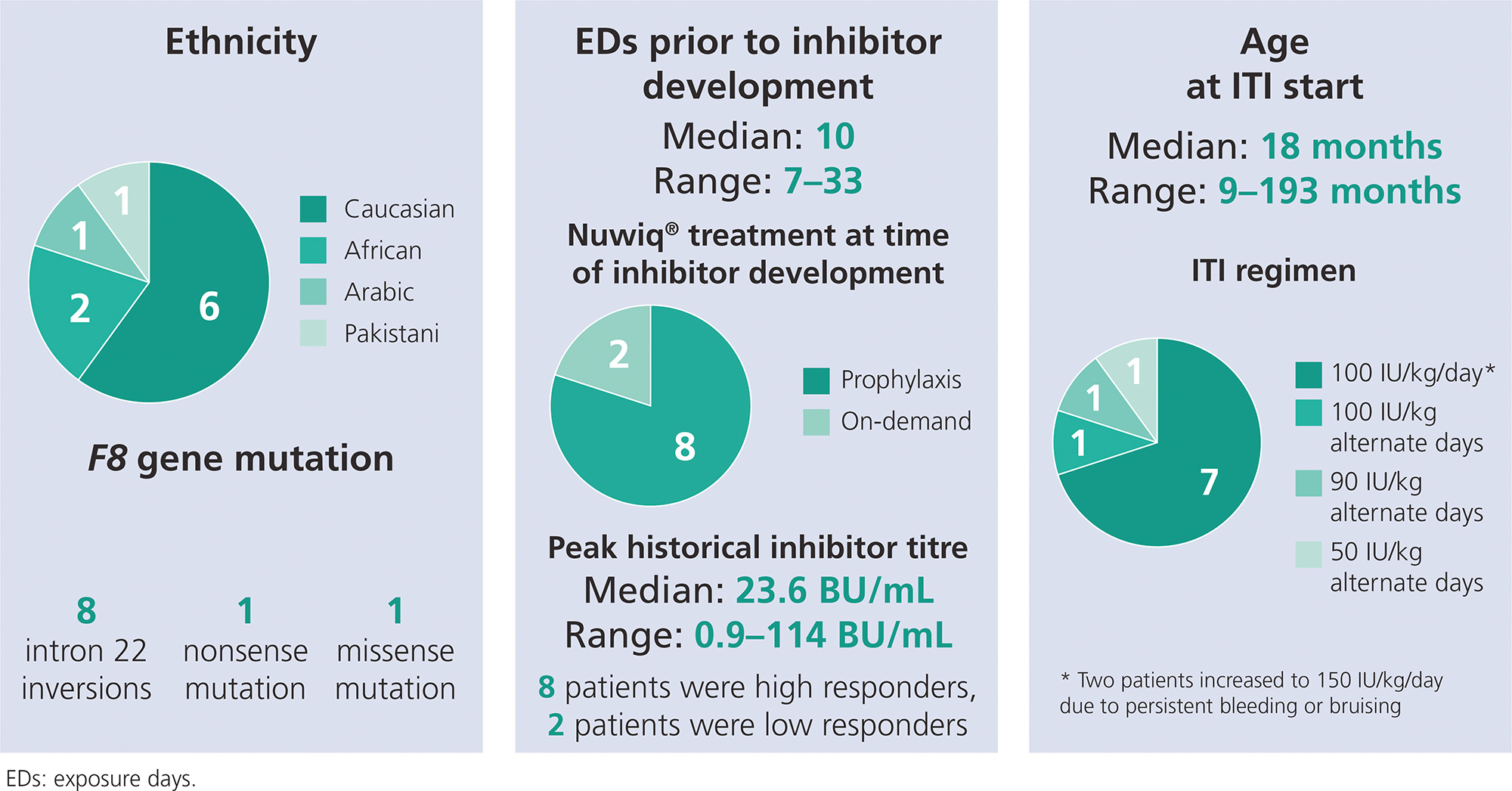
Figure 1. Patient demographics and clinical characteristics
ITI outcomes
- ITI outcomes for the 10 patients are shown in Table 2
- Inhibitors have been eliminated in 8 of 10 (80%) patients
- 1 patient discontinued ITI with simoctocog alfa after 14 months due to an increasing inhibitor level; this patient had several poor prognostic factors and was first treated at the age of 15 years and had poor joint health, peak inhibitor titre > 100 BU/mL, and was Black African ethnicity
- 8 patients have achieved success so far
- 2 patients (both high responders) achieved complete success after 7 and 9 months
- 6 patients have achieved partial success; 4 continue to receive ITI with simoctocog alfa
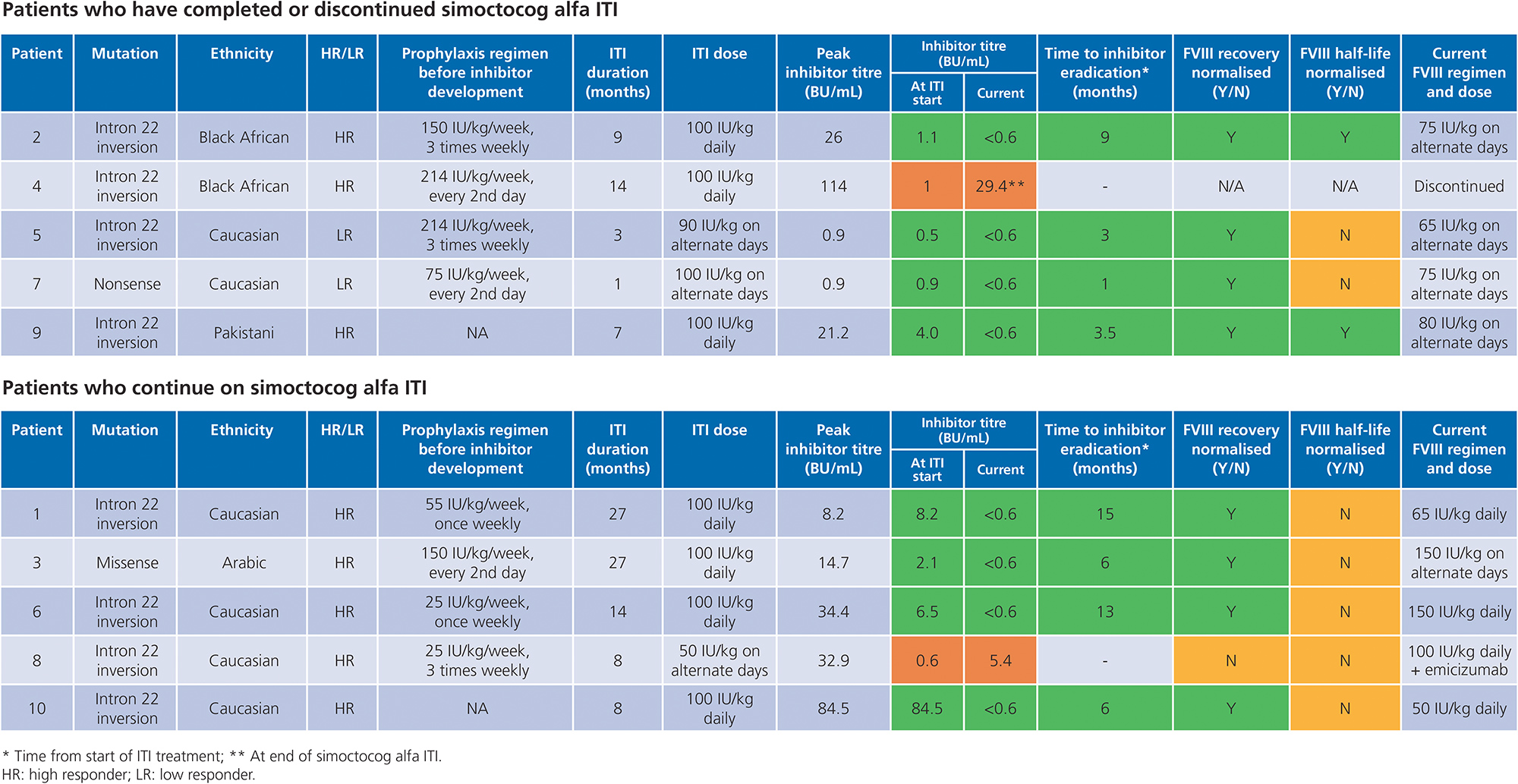
Table 2. ITI outcomes
Bleeding, tolerability and safety
- 6 of the 10 patients (60%) experienced no bleeds during ITI with simoctocog alfa
- 3 patients experienced 1 bleed, and 1 patient 2 bleeds, which were treated with recombinant FVIIa
- Simoctocog alfa was well-tolerated, with no adverse drug reactions reported
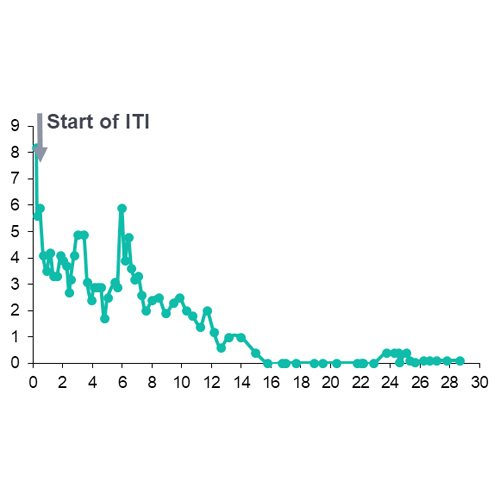
- Progress of inhibitor eradication over time in individual patients is shown for the 9 patients with data available in Figure 2
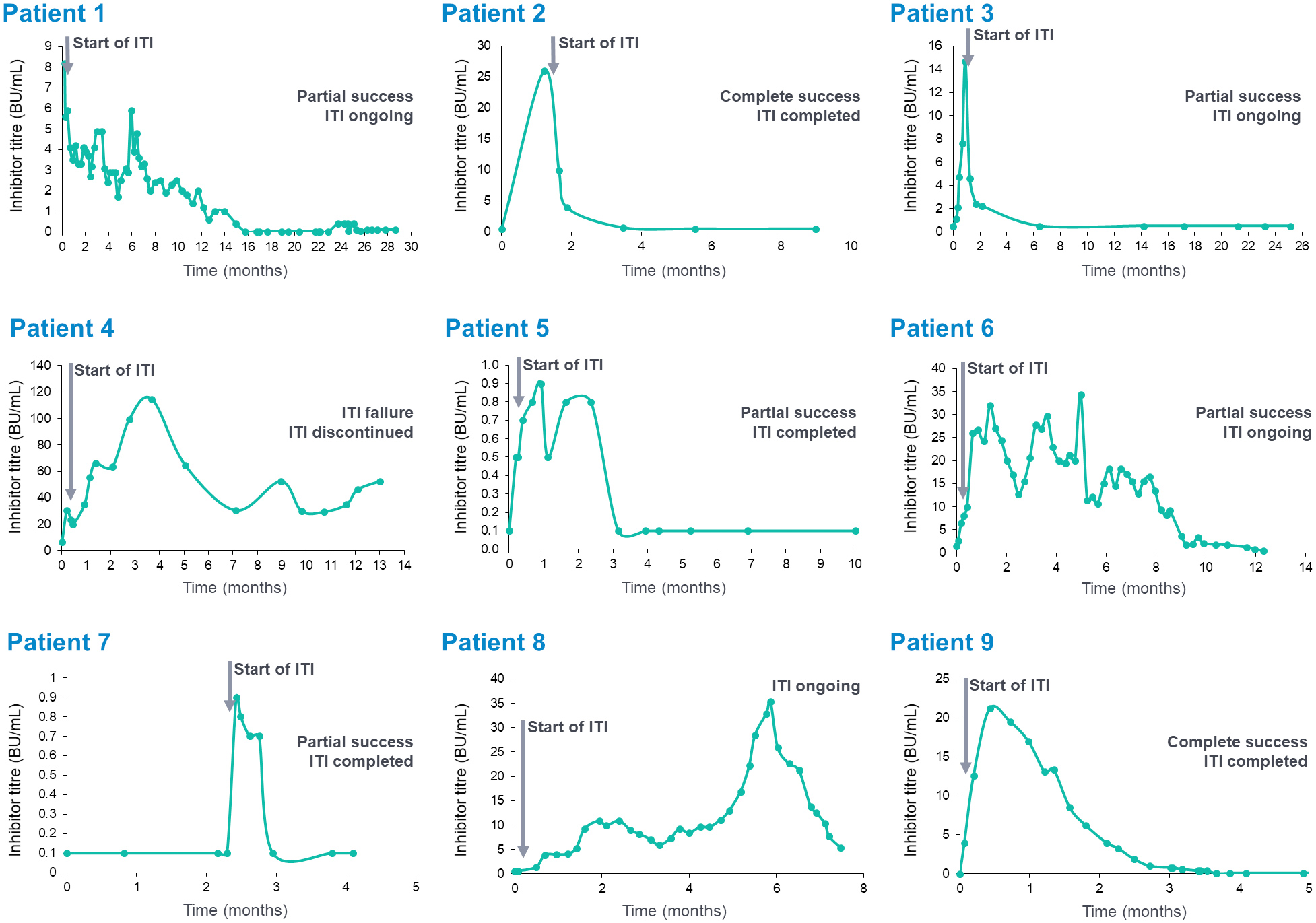
Figure 2. Inhibitor levels over time in individual patients
Conclusions
- Simoctocog alfa (Nuwiq®) has been used for ITI in 3 centres in the UK in 10 patients with severe haemophilia A who developed inhibitors to simoctocog alfa
- Success criteria were defined as an undetectable inhibitor titre (< 0.6 BU/mL), FVIII recovery of
≥ 66% and FVIII half-life of ≥ 6 hours - Inhibitors have been eradicated in 8 of 10 (80%) patients so far
- 8 patients achieved success
- 2 patients achieved complete success after 7 and 9 months
- 6 patients achieved partial success; 4 continue to receive ITI
- Simoctocog alfa was well-tolerated, with no adverse drug reactions reported
References
1. Mizoguchi Y et al. Int J Hematol 2016; 103:473–7.
2. Valentino L et al. Haemophilia 2015; 21:559–67.
3. Liesner R et al. Haemophilia 2018; 24:211–20.
4. Hay C et al. Blood 2012; 119:1335–44.