Rauch A.1,2, Paris C.1, Repesse Y.3, Borel-Derlon A.3, Rugeri L.4, Harroche A.5, Ternisien C.6, Castet S.7, Lebreton A.8, Pan-Petesch B.9, Volot F.10, Clayssens S.11, Chamouni P.12, Gay V.13, Berger C.14, Desprez D.15, Biron-Andreani C.16, Veyradier A.17, Goudemand J.1, Susen S.1,2 for the French Reference Center of von Willebrand Disease
1CHU Lille, Hematology Transfusion, Lille, France; 2INSERM, U1011, University of Lille, U1011-EGID, Institut Pasteur de Lille, Lille, France; 3Laboratoire d’hématologie, CHU de Caen, Caen, France; 4CHU Lyon, Unité d’Hémostase Clinique/Centre Régional de Traitement de l’Hémophilie, Bron, France; 5Hôpital Necker Enfants Malades APHP, Paris, France; 6CHU Nantes, Laboratoire d’Hématologie, Nantes, France; 7CHU Bordeaux Pellegrin, Bordeaux, France; 8CHU Clermont Ferrand, Clermont Ferrand, France; 9CHU Brest, Brest, France; 10CHU Dijon, Dijon, France; 11CHU Toulouse, Toulouse, France; 12CHU Caen, Rouen, France; 13CH Métropole Savoie-Site de Chambéry, Chambéry, France; 14CHU Saint-Etienne, St Etienne, France; 15CHU Strasbourg, Strasbourg, France; 16CHU Montpellier, Montpellier, France; 17GH St-Louis Lariboisière F. Widal – Hôpital Lariboisière APHP, Laboratoire d’Hématologie, Paris, France
METHODS
- A 15-items mail-based survey was first sent to 30 centers between December 2017 and February 2018 to identify constitutional VWD-patients referred for at least one GIB episode (including hematemesis, melena, hematochezia or unexplained chronic iron deficiency anemia) between January 2015 and January 2018. All centers had access to a digestive intensive care unit and to the full set of endoscopic exploration including video capsule endoscopy (VCE).
- Only constitutional VWD-patients fulfilling at least one of the following CRMW laboratory inclusion criteria* were considered for analysis. Any patient with a clinical context potentially responsible for an acquired von Willebrand syndrome was thus excluded.
* for type 1 phenotype, VWF levels <30IU/dL (together with VWF:RCo/VWF:Ag and FVIII:C/VWF:Ag ratios >0.6); for type 2 phenotype, a decreased or normal VWF levels with a discrepancy between the antigenic and the functional levels (VWF:RCo/VWF:Ag or FVIII:C/VWF:Ag ratios < or = 0.6); and for type 3 phenotype, VWF plasma levels <5 IU/dL.
POPULATION
- A total of 21 centers (70%) completed the survey. During the 3-years follow-up period, a total of 137 GIB-episodes (mean = 0.85/patient-year) were reported in 54 VWD-patients. At the start of the follow-up, 27 patients had an history of GIB (50%) including 9 patients (16%) with a previous GIAD diagnosis. 7 of these 9 GIAD-patients had a VWD2A (n=2), 2B (n=3) or 3 (n=2). 7 patients were under long-term prophylaxis with VWF concentrates at the start of the follow-up. Data on endoscopic exploration and GIB management were available for 46 patients
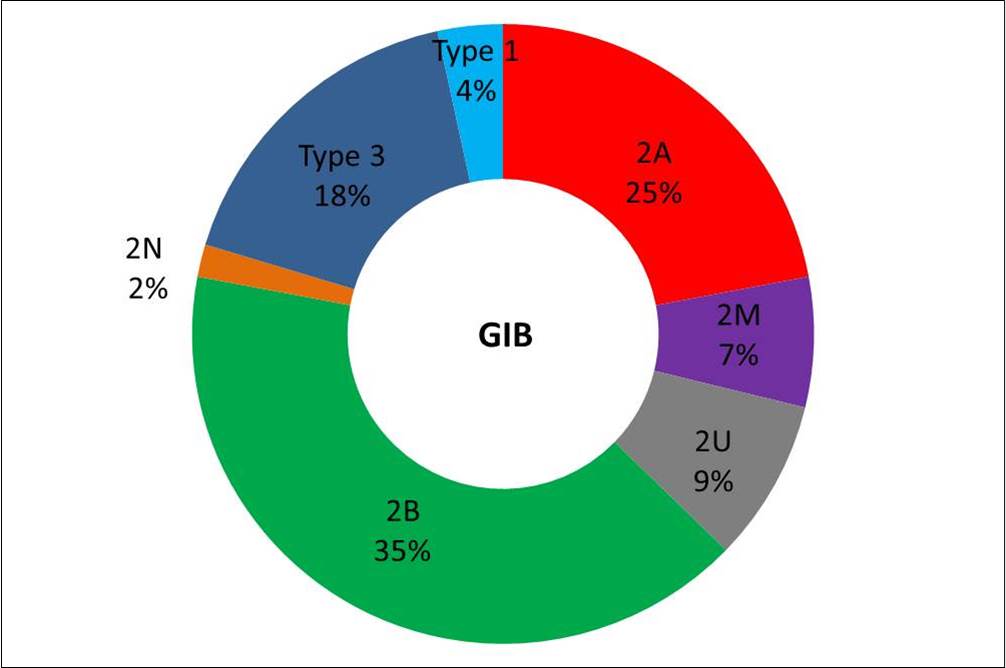
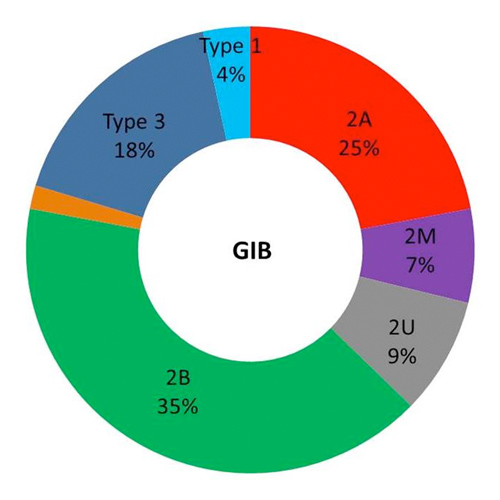
- VWD2A, 2B and 3 were more frequently associated with GIB (78% of patients; 88% of episodes) (Fig 1). GIB occurred at a significantly younger age in VWD2A, 2B and 3 patients when compared to other subtypes, even after exclusion of type 3 patients (p< 0.01). GIB pediatric cases were only observed in VWD2A (n=2) and 3 (n=2).
ENDOSCOPIC MANAGEMENT OF GI BLEEDING
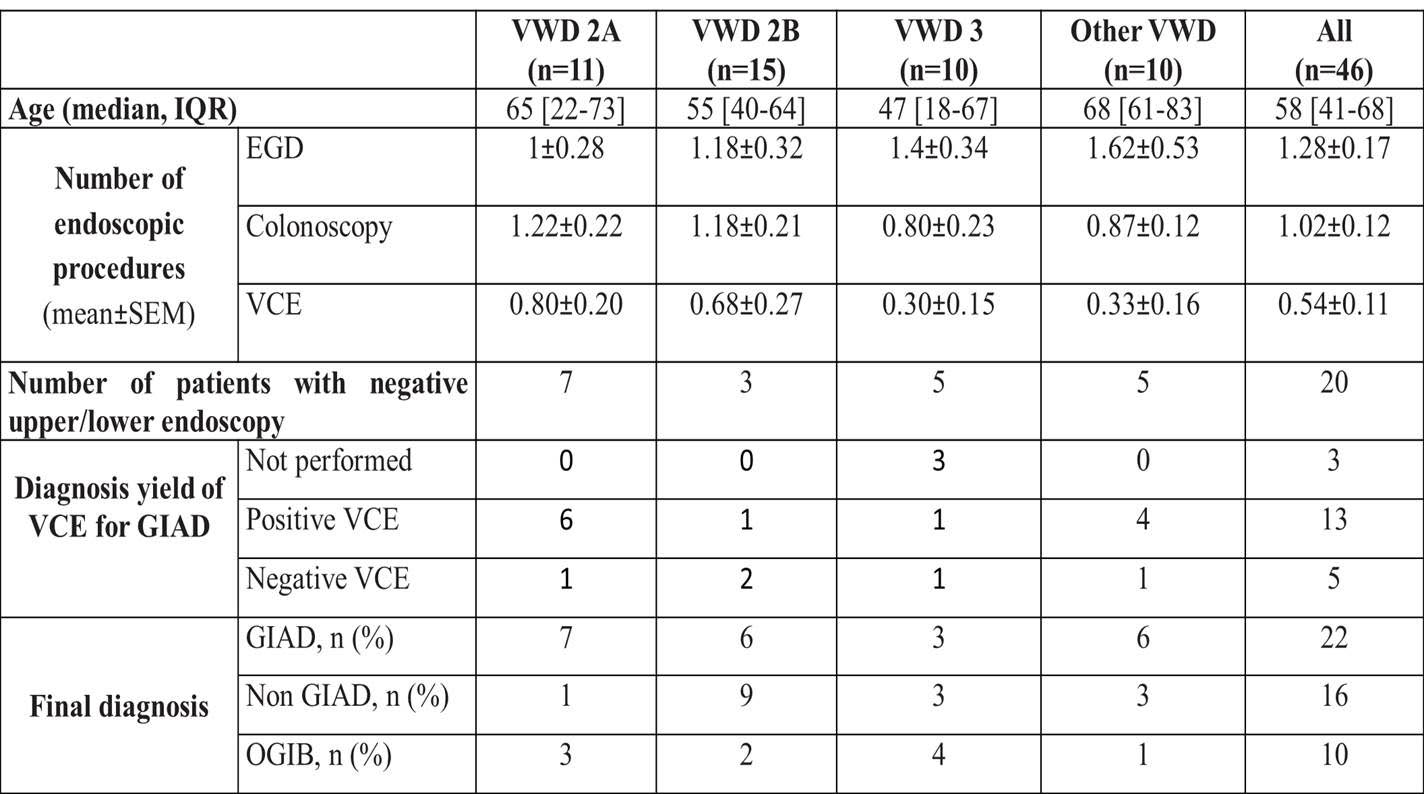
- In patients without bleeding source identified after conventional endoscopy (esophagogastroduodenoscopy, EGD & colonoscopy), a videocapsule endoscopy (VCE) was performed at first GIB episode or at first GIB recurrence in 40% and 70% of cases, respectively.
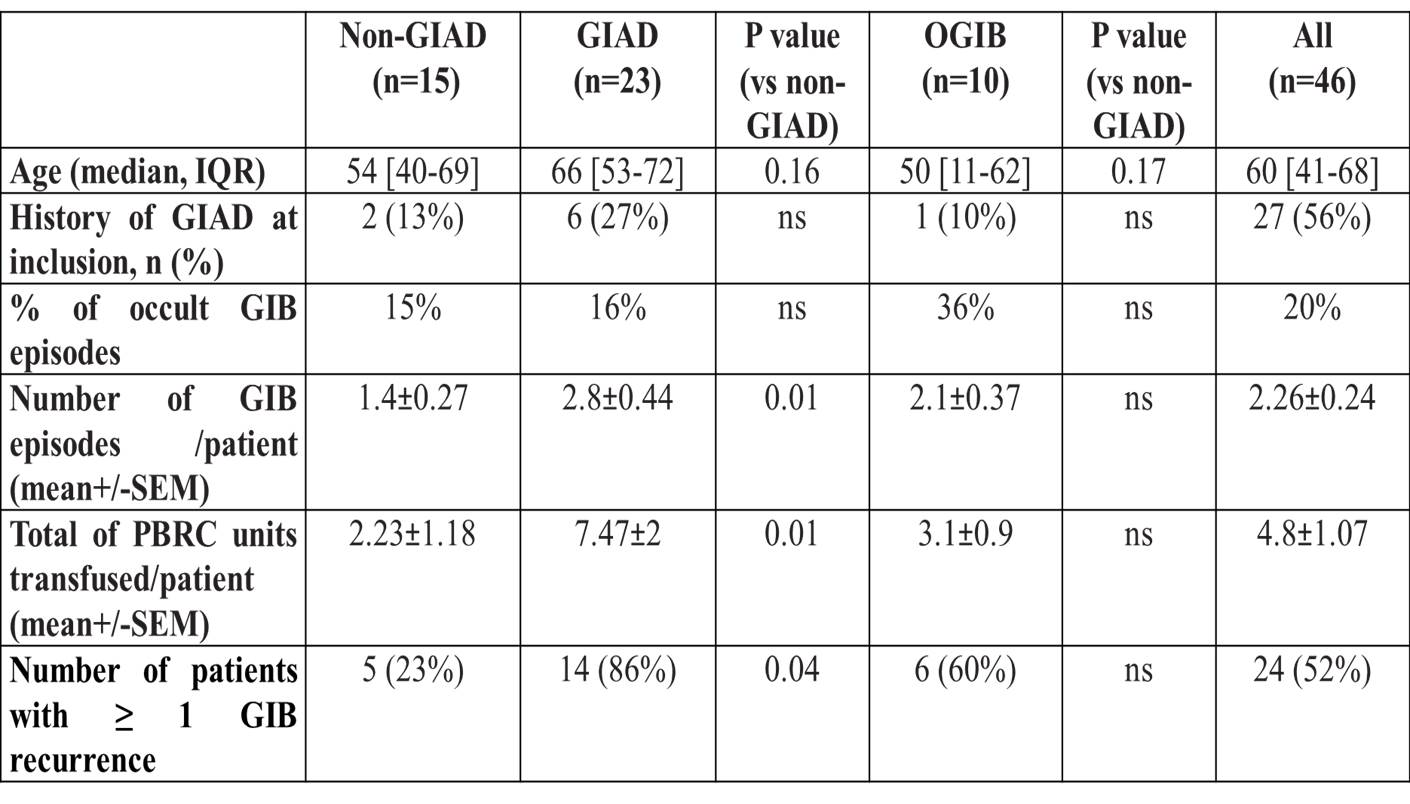
- At the end of the follow-up, GIAD were identified in a total of 22 patients, of whom 15 were VWD2A, 2B or 3. During the follow-up, the yield of VCE for GIAD diagnosis in the 20 VWD patients with negative findings after EGD and colonoscopy was 72%. Common causes of GIB were identified in 15 patients through EGD or colonoscopy (non-GIAD group). Uptake of NSAIDs, anti-platelet drugs or vitamin K antagonist were also identified in 6 patients. Endoscopic exploration remained negative in 10 patients therefore categorized as “occult GIB” (OGIB). 9 off these OGIB-patients had a VWD2A, 2B or 3 (Table 1). No GIAD was identified in the 4 pediatric cases 3 of them were classified as OGIB. GIAD patients were more likely to have GIB recurrence (p<0.05) and had higher transfusion needs (p<0.01) compared to non-GIAD patients (Table 2).
THERAPEUTIC MANAGEMENT OF GI BLEEDING
- During the follow-up, 60% of GIAD (n=15) patients underwent at least one session of endoscopic Argon Plasma Coagulation.
- Long-term prophylaxis with VWF concentrates was introduced in a total of 14 patients (26%) for either GIB-related to GIAD (n=8) or to OGIB.
- An off-label use of antiangiogenic drugs was also reported in 6 GIAD-patients.
CONCLUSION
- This nationwide retrospective study outlines that GIAD represent a common cause of GIB in VWD-patients.
- There is a high inter-centers heterogeneity in endoscopic exploration notably regarding the use and timing of VCE.
- There is a high inter-centers heterogeneity in the management of GIAD and OGIB-related GIB in VWD-patients
INTRODUCTION
- Gastrointestinal bleeding (GIB) has been reported as the first cause of hospitalization in congenital Von Willebrand Disease (VWD) with frequent recurrences even under VWF prophylaxis at least in a subset of patients with gastrointestinal angiodysplasia (GIAD).
- Guidelines for exploration and treatment of GIB in VWD-patients are lacking.
AIM
To evaluate over 3 years the current practice concerning GIB exploration and treatment among the network of French comprehensive care centers for VWD.