M. Llusá, M. Griffiths, N. B. Binder
Research and Development Department, Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH, Vienna, Austria
Introduction
The aim of these studies was to develop a fully automated ADAMTS13 activity assay using a fluorescence resonance energy transfer (FRET) method. For this purpose a new coagulation analyzer, equipped with an optical Quenching module was co-developed. Generally, the new ADAMTS13 activity assays should benefit from an automated analytical process (eliminating errors, improve quality, reduce work load, turn-around time and costs) while maintaining the key performance parameters of the well established TECHNOZYM ADAMTS-13 Activity ELISA (LOQ, linearity) to ensue patient safety.
Materials and Methods
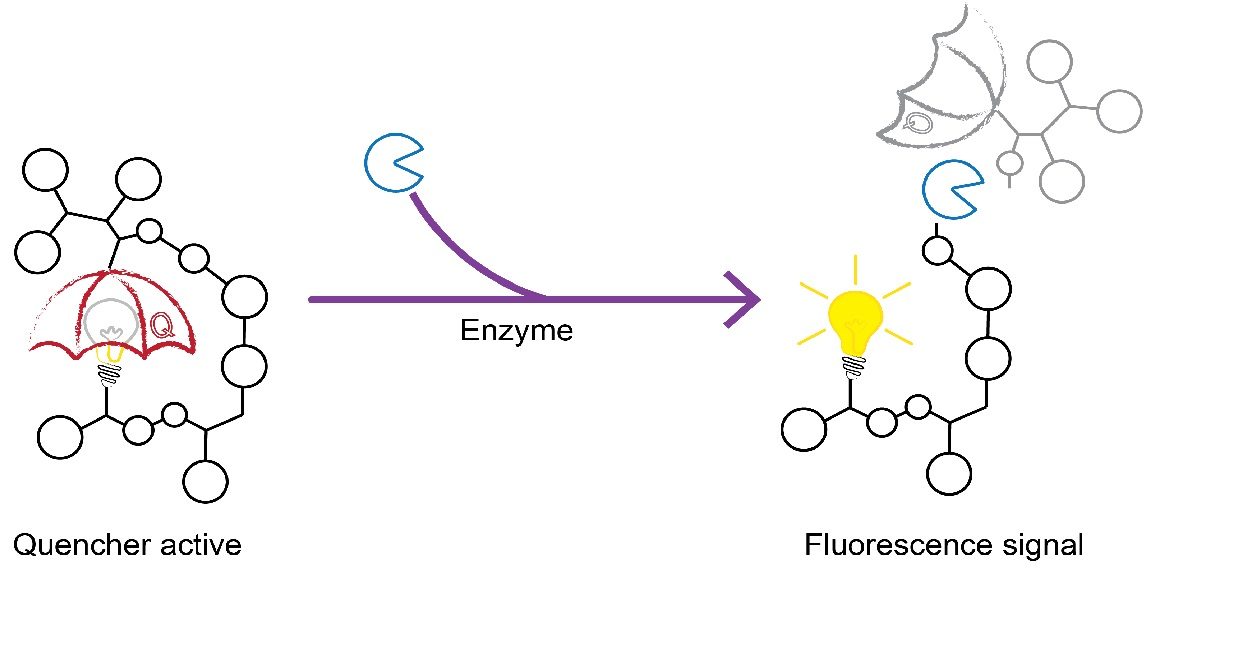
Assay principle
The newly developed TECHNOFLUOR ADAMTS13 Activity employs a FRET substrate based on the VWF73. Upon cleavage by sample derived ADAMTS13, the quenching part is removed and flourescent signal is emmitted. The measurable signal is proportional to the ADAMTS13 activity level.
A newly developed automated analyzer with Quenching module (Ceveron s100), combining routine and speciality haemostasis testing, was used.

Results
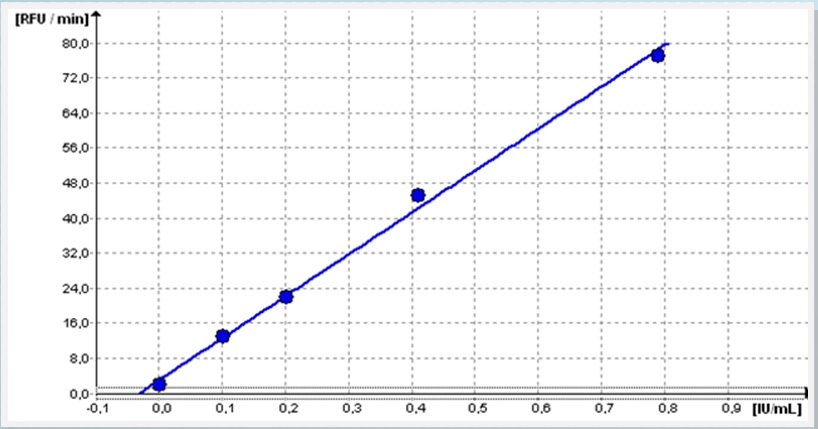
Calibration
With an assay time of <30 min a calibration curve ranging from 0 – 0.8 IU/mL could be established exhibiting excellent recovery of controls being calibrated with TECHNOZYM ELISA system.
High Control (0.71 IU/mL) – 101% recovery
Low Control (0.16 IU/mL) – 94% recovery
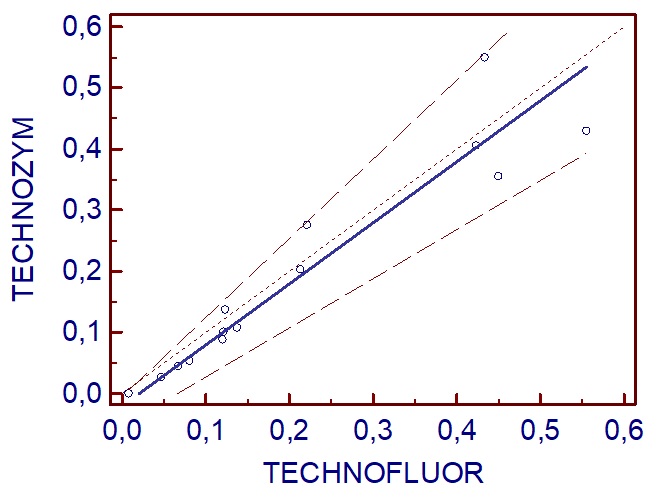
Method comparison
The linear correlation (Passing and Bablok regresion) between TECHNOZYM ELISA results and the new automated method (TECHNOFLUOR) shows a good correlation coefficient (r>0.90) and an intercept of <0.01 IU/mL.
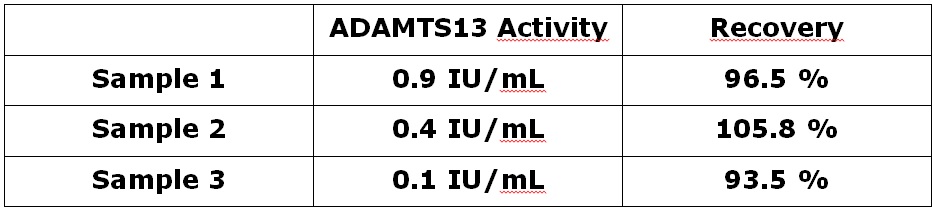
Stability
To investigate the stability of the new ADAMTS13 activity substrate three samples with different activity levels were tested. Lyophilized substrate was compared fresh and stored for 1 month at 37°C.
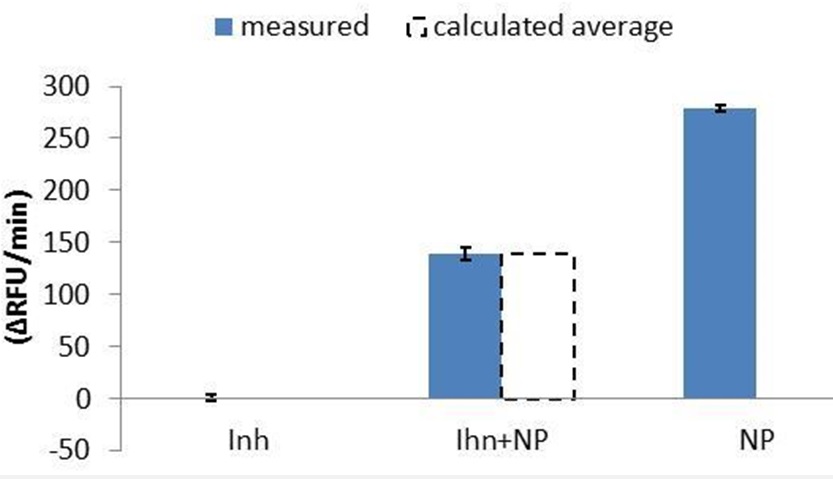
Effect of inhibitors
Analyzing an automated mixing study for ADAMTS13 we studied an EQA sample from ECAT (18 INH units). Although the measured activity was <0.1 IU/mL, the sample mixed with normal pooled plasma (1+1) did not show effects of the ADAMTS13 inhibitors.
Conclusion
The fully automated ADAMTS13 Activity test run on the new Ceveron s100 haemostasis analyzer is a fast, easy to use and accurate option to improve ADAMTS13 testing for faster diagnostics and better treatment of TMA patients. With a high degree of linearity over the whole assay range, the new TECHNOFLUOR ADAMTS13 Activity assay shows excellent comparison to both reference methods for ADAMTS13 (FRETS and chromogenic ELISA).
Based on the stability experiments we expect a minimum shelf life of 24 months. The use of lyophilized INH samples might be problematic (known incompatibility for Bethesda-like test systems) and further studies need to test fresh or frozen samples.
Technoclone Herstellung von Diagnostika und Arzneimitteln GmbH ♦ Brunner Str. 67 ♦1230 Vienna ♦ Austria ♦
Tel: +43 1 86 373 0 ♦ Fax: +43 1 86 373 44 ♦ Email: products@technoclone.com
