Aisina R.B.1, Mukhametova L.I.1,2, Ivanova E.M.1
1Lomonosov Moscow State University, Chemistry Department, Moscow, Russia; 2Bauman State Technical University, Moscow, Russia
Background
Polyamidoamine (PAMAM) dendrimers, monodispersed polymers with highly branched three-dimensional globular structure and surface functional groups (-NH2, -OH, or -COOH), are of growing interest in nanomedicine, notably in systemic administrations as drug carriers. Charged surface groups of PAMAM dendrimers can to form non-covalent complexes with drugs, and are available for covalent attachment of biomacromolecules. PEGylation of FVIII doubles its lifetime in bloodstream while maintaining procoagulant activity. Modification of FVIII with PAMAM-dendrimers of various generation and surface charge can be used to obtain conjugates with desired properties.
Aim
To study the effect of PAMAM-NH2 dendrimers (G1-G3) and PAMAM-COOH (G1.5-G3.5) on blood coagulation components to select optimal polymer for modifying target factors.
Materials and Methods
Effect of the PAMAM-dendrimers concentration on RBC hemolysis, overall hemostatic potential, thrombin generation and activity, prothrombin time, zeta potential and CD spectrum of fibrinogen was studied.
Results
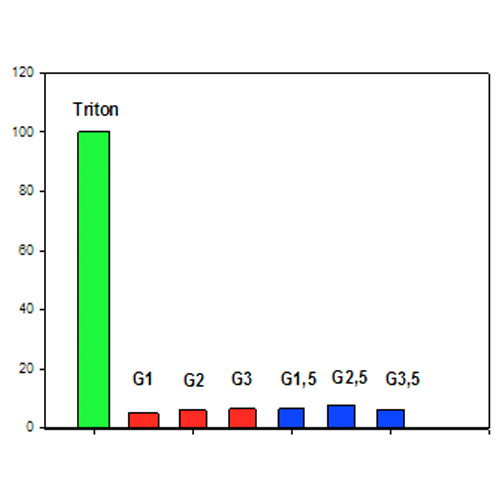
Fig. 1. The influence of PAMAM dendrimers on hemolysis level
We compared hemolysis of RBCs induced by cationic and anionic PAMAM dendrimers of low generation by measuring released hemoglobin. The PAMAM-NH2 (G1-G3) and PAMAM-COOH (G1.5-G3.5) dendrimers at high concentration (600 µM) were used to measure their effects on RBCs after 4 h. We found that the degree of RBC hemolysis induced even by a high concentration of all the studied dendrimers is only about 5-8%.
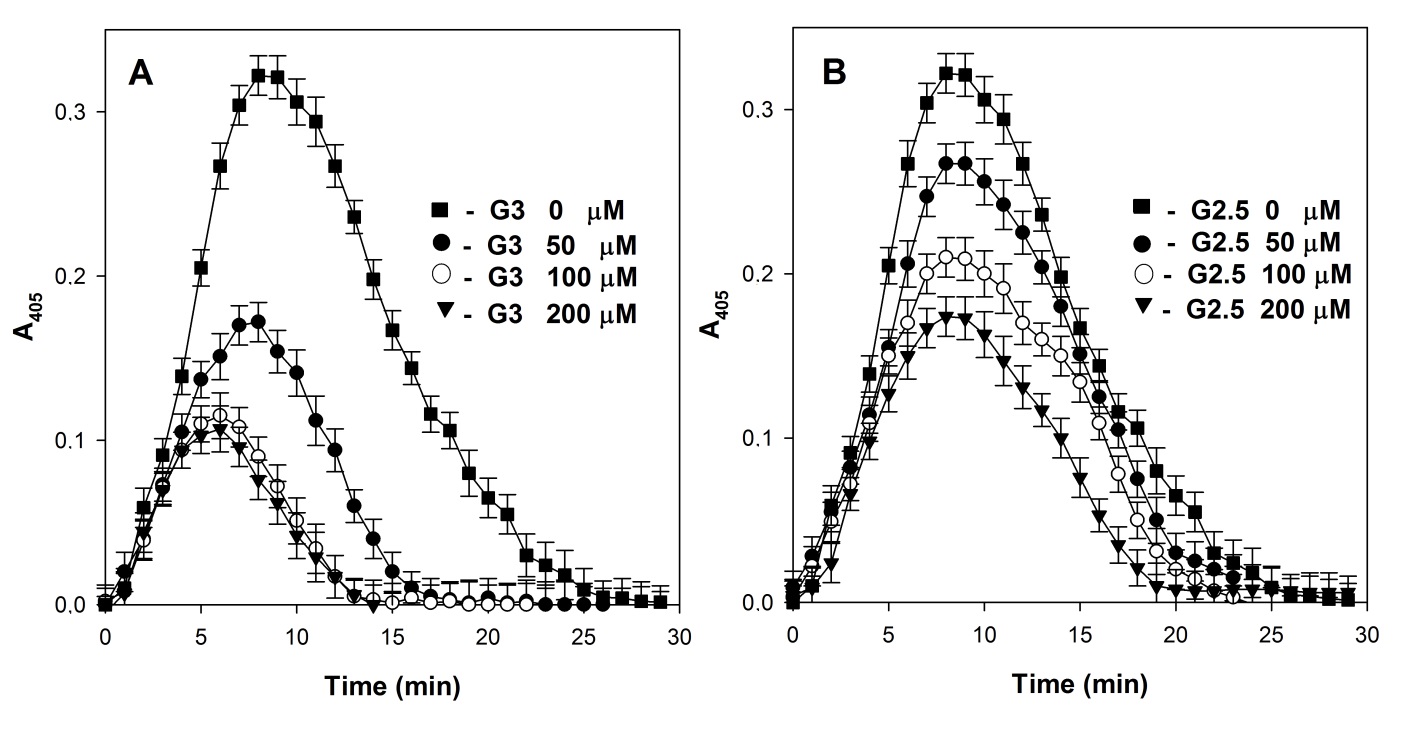
Fig. 2. Influence of concentration of G3 PAMAM (A) and G2.5 PAMAM (B) dendrimers on overall hemostatic potential of plasma.
PAMAM dendrimers G3 and G2.5 have the same diameter (3.6 nm) and number of charged surface groups (32). Fig. 2 presented their influence on the plasma fibrin time curves in the presence of exogenous thrombin and tPA. Increase of the concentration of cationic G3 dendrimer reduced significantly the coagulation rate of plasma and the maximum amount of clot (peak height) induced by added thrombin, resulting in a decrease in fibrinolysis by added tPA (Fig. 1A). Anionic G2.5 dendrimer on the fibrin time curve was much smaller than that of cationic G3 dendrimer (Fig. 1B). Hence, a more significant decrease in the overall hemostatic potential of the plasma (total area under the curve) caused by the G3 dendrimer compared to the G2.5 dendrimer is associated only with its positive surface charge.
The observed PTT elongation in human plasma in the presence of <10 μM G3 PAMAM dendrimer concentrations (data was not shown) are probably caused by the prevention of the procoagulant protein binding and the prothrombinase complex formation on the phospholipid matrix.I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
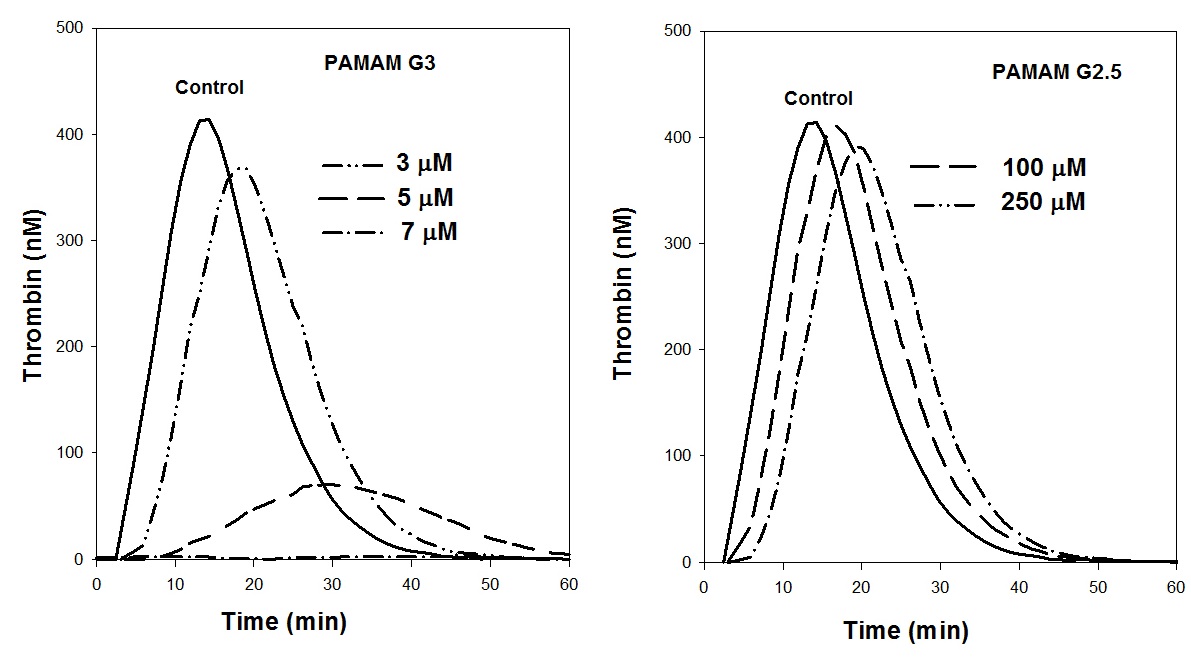
Fig. 3. Influence of concentration of G3 PAMAM and G2.5 PAMAM dendrimers on thrombin generation in plasma by rTF (37°C).
Fig 3 shows the effect of concentration of the G3 and G2.5 PAMAM dendrimers on the profile of endogenous thrombin generation by rTF. The cationic G3 PAMAM dendrimer very strongly inhibits the generation of endogenous thrombin. Compared with control curve, the presence of this dendrimer even at concentration of 5 µM increases the lag phase 2.4-fold and time-to-peak by 2-fold and decreases the maximum concentration of thrombin by 8-fold. The generation of endogenous thrombin was completely suppressed by 7 µM concentration of this dendrimer.
The effect of the anionic PAMAM G 2.5 dendrimer was negligible even at concentrations of more than 250 mM.
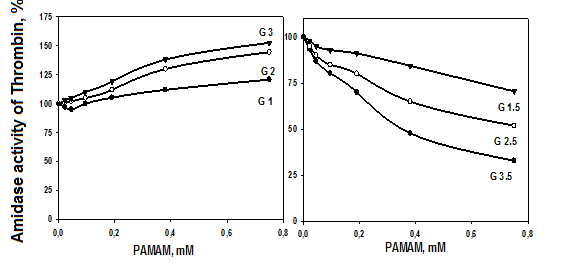
Fig. 4. Amidolytic activity of thrombin (5 nM) depending on the concentration and generation of PAMAM-NH2 (G1-G3) and PAMAM-COOH (G1.5-G3.5) dendrimers. The hydrolysis rates of specific chromogenic substrate by thrombin in 0.1 M PBS buffer (pH 7.4) containing various concentrations of dendrimers were measured at 405 nm and 25°C.
Fig. 4 shows that the dependences of the amidolytic activities of thrombin on the concentration of PAMAM-NH2 and PAMAM-COOH dendrimers. The intrinsic activities of thrombin were determined using the hydrolysis of its specific substrates Z-Ala-Ala-Arg-pNA. A slight increase in the activities of thrombin was observed with increasing concentration (up to 300 µM) and generation of cationic (G1-G3) dendrimers. The G1-G3 dendrimers do not interfere with the ability of thrombin to cleave its chromogenic substrate, suggesting that the active sites of thrombin retain the ability to cleave protein substrates, such as fibrinogen and plasminogen. On the contrary, anionic (G1.5-G3.5) PAMAM dendrimers had a marked inhibitory effect on the activities of thrombin which increased with increasing concentration and generation of the dendrimers.
Perhaps the interaction of thrombin with low-generation of cationic PAMAM dendrimers causes some conformational changes in the enzymes that affect their active centers. The binding of cationic dendrimers to negatively charged centers of thrombin can somewhat stabilize the conformation of enzymes, which leads to a slight increase in their activity.
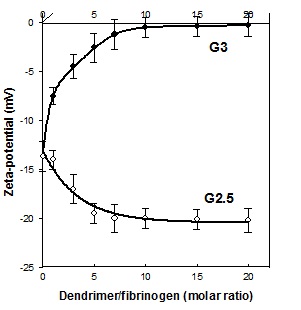
Fig. 5. Zeta potential of fibrinogen (4 µM) in the presence of increasing concentrations of G3 and G2.5 PAMAM dendrimers in 10 mM phosphate buffer, pH 7.4 (25°C).
The zeta potential of pure fibrinogen is negative and has a mean value of –17.65 mV. Fig. 5 shows the changes in the zeta potential of fibrinogen in the presence of increasing concentrations of G3 and G2.5. Addition of G3 dendrimer with amine functional groups led to gradual changes in the zeta potential towards positive values up to dendrimer/protein molar ratios of 10:1. After addition of G2.5 dendrimer with carboxyl functional groups, this value became more negative up to dendrimer/protein molar ratios of 7:1. Graphs of the zeta potential versus the molar dendrimer/fibrinogen ratio show that at least 10 molecules of G3 PAMAM-NH2 and 7 molecules of G2.5 PAMAM-COOH can bind to one fibrinogen molecule. A strong change in zeta potential of fibrinogen towards positive values after binding about 10 molecules of the G3 dendrimer and a weaker its change towards negative value after binding about 7 molecules of the G2.5 dendrimer.
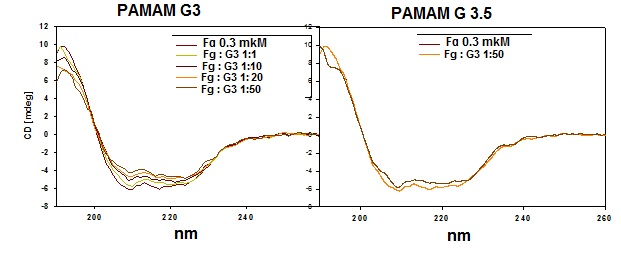
Fig. 6. The influence of different concentration of PAMAM G3 and G3.5 on CD-spectra of human fibrinogen.
Fig. 6 presents the results of study of influence of different concentrations of PAMAM dendrimers G3 and G3.5 on the CD spectra of fibrinogen 0.3 mkM. An increase in the concentration of the cationic PAMAM G3 dendrimer leads to changes in the spectrum at 208 and 222 nm, which indicates on change in the content of the α-helix in the fibrinogen molecule due to its interaction with the cationic dendrimer. On the contrary, the increase in the concentration of anionic dendrimer G2.5 does not cause changes in the spectrum: the spectra of fibrinogen in the presence of high concentrations of PAMAM G3.5 is almost identical with the spectrum of the native protein. Therefore, anionic dendrimer G3.5 practically does not affect the structure of the molecule of fibrinogen.
Conclusions
The cationic dendrimers (G1-G3) increase the prothrombin time and suppress the generation of endogenous thrombin, while anionic dendrimers have no effect. Compared to anionic dendrimers, cationic dendrimers inhibited more strongly the overall hemostatic potential in plasma. The cationic dendrimers, strongly binding to negatively charged fibrinogen, alter the conformation and coagulability of the latter. Anionic dendrimers do not affect the kinetics of fibrinogen polymerization by thrombin. Our results demonstrate that PAMAM-NH2 dendrimers inhibit the extrinsic activation pathway of the coagulation system and change conformation and function of fibrinogen. Anionic PAMAM dendrimers, not cationic dendrimers, are promising for modification of the factor FVIII amino groups, because unlike linear PEG, compact dendrimer molecules do not cover the protein, but can block its degradable bonds and antigenic epitopes.